Process for degrading zearalenone in a feed product employing laccase
A technology of zearalenone and products, applied in the field of feed products detoxified by mycotoxin zearalenone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
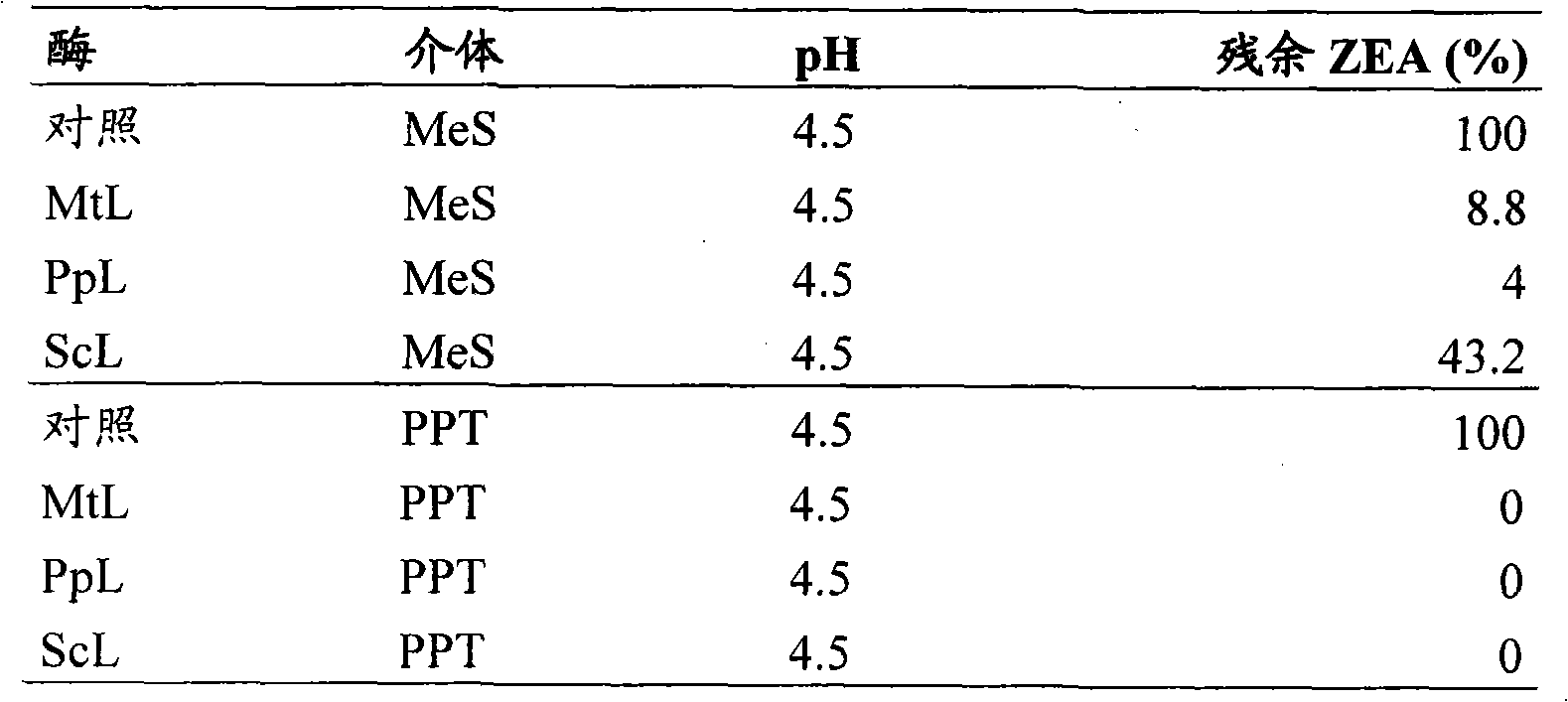
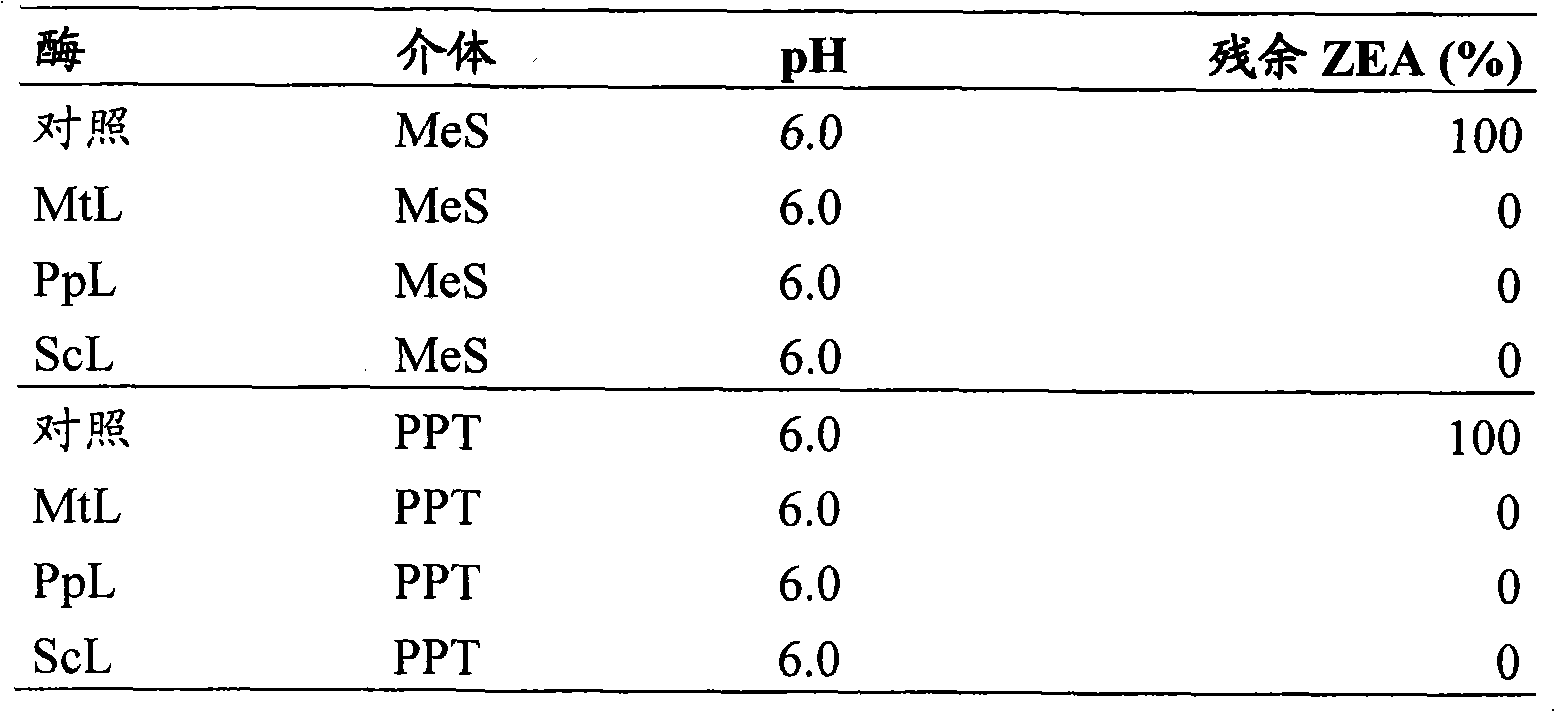
[0061] Materials and methods
[0062] enzyme
[0063] Laccase from Myceliophthora thermophila (MtL) having the amino acid sequence set forth herein as SEQ ID NO:1.
[0064] Laccase from Polyporus pinsitus (PpL) having the amino acid sequence shown herein as SEQ ID NO:2.
[0065] Laccase from Streptomyces coelicolor (ScL) having the amino acid sequence set forth herein as SEQ ID NO:3.
[0066] mediator
[0067] Methyl syringate (MeS)
[0068] Phenothiazine-10-Propionic Acid (PPT)
[0069] Assay : Reactions were performed in Eppendorf tubes in a volume of 300 μl containing zearalenone 30 μM, mediator 0.2 mM, sodium acetate 100 mM and enzyme 0.1 mg EP / mL. In a control reaction, the enzyme-containing volume was replaced with an equal amount of H 2 O substitute. The reaction was incubated at 37°C for 24 hours, after which the reaction was stopped by adding 600 μL of 100 μM acetonitrile stop solution. The reaction was stored at -20°C until chromatographic analysis.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com