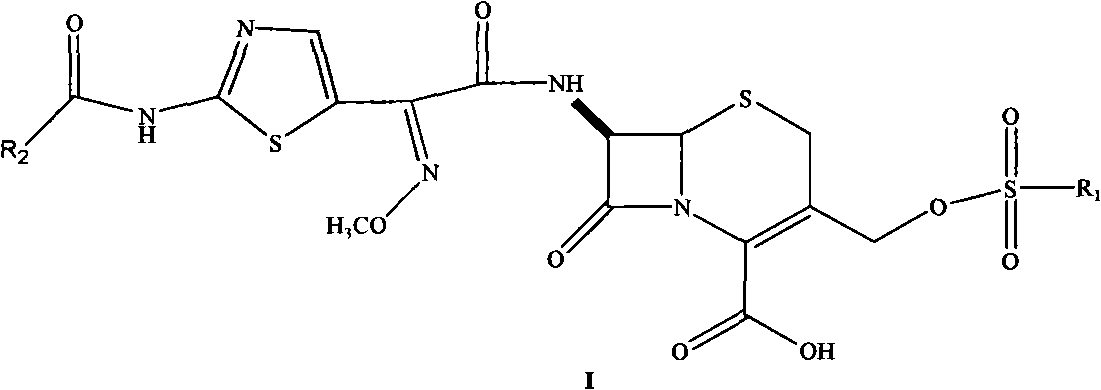
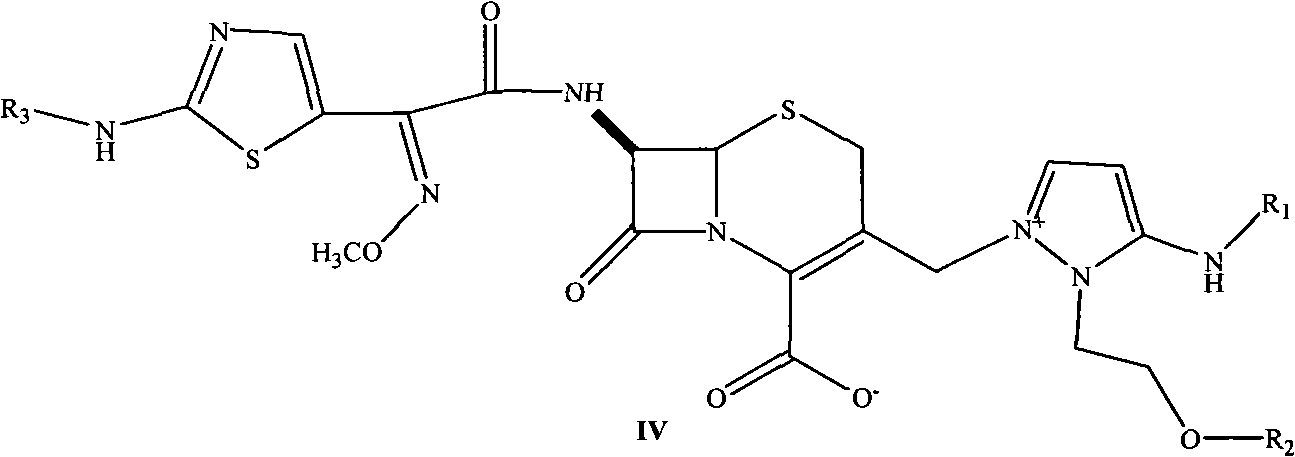
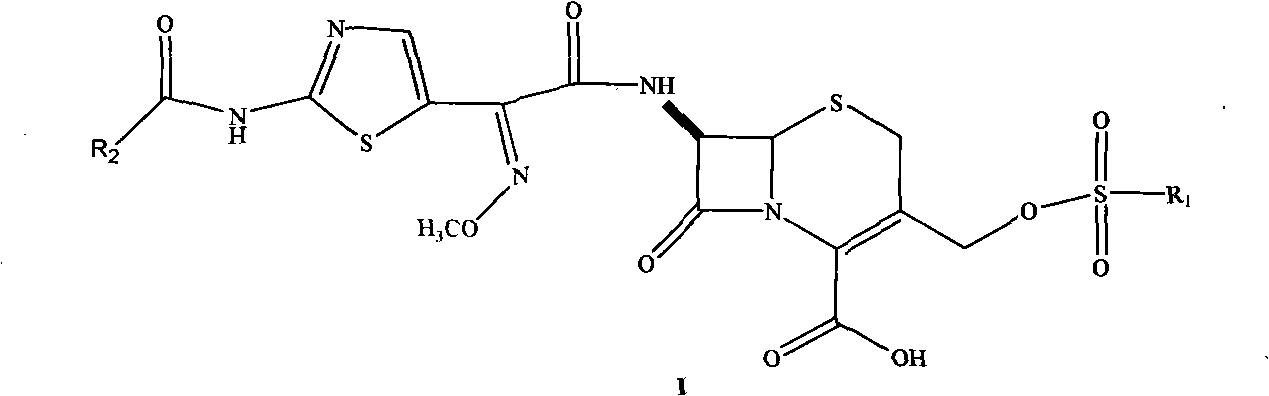
Cephalosporin nucleus derivative compound, cephaene onium salt compound prepared from same, and method for preparing cefpiramide sulfate from cephalosporin nucleus derivative compound and cephaene onium salt compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, the first step: the synthesis of 7β-amino-3-hydroxyl-3-cephalosporin-4-carboxylic acid (II): 20 milliliters of methyl alcohol is loaded into a 100 milliliter three-necked flask, 20 milliliters of water, add 6 grams of 7- ACA, cooled to -40°C, added dropwise 1.76 g of sodium hydroxide in 5 ml of aqueous solution, after the addition, kept stirring for 30 min, adjusted the pH to 1-2 with 15% hydrochloric acid, stirred for 2 h, suction filtered, and filtered the cake with water Washing and vacuum drying at 40°C gave 4.5 g of a white solid with a yield of 90% and a purity of 98.7%;
[0041] 1 H-NMR (DMSO-d 6 )δ3.45 (1H, d, J = 18Hz), 3.55 (2H, d, J = 18Hz), 4.18 (1H, d, J = 13.6Hz), 4.23 (1H, d, J = 13.6Hz), 4.72 ( 1H, d, J = 4.8Hz), 4.92 (1H, d, J = 4.8Hz), 6.25 (2H, brs)
[0042] The second step: 7β-[2-(2-formylaminothiazol-4-yl)-2-(methoxyiminoacetamide)]-3-hydroxyl-3-cephem-4-carboxylic acid (III ) synthesis: put 40 ml tetrahydrofuran and 20 ml water in...
Embodiment 2
[0050] Embodiment 2, the first step is with embodiment 1;
[0051] The second step: 7β-[2-(2-formylaminothiazol-4-yl)-2-(methoxyiminoacetamide)]-3-hydroxyl-3-cephem-4-carboxylic acid (III ) synthesis: 30 milliliters of acetonitrile and 20 milliliters of water are loaded into a 200 milliliter three-necked flask, 4 grams of compound (II) synthesized in the first step are added, saturated sodium bicarbonate solution is added dropwise to pH=7-8, cooled to 0- Add 4.0 grams of active ester in batches at 5°C, while adding, use saturated sodium bicarbonate solution to control pH=7-8, after the addition, slowly heat up to about 40°C, stir for 6 hours, filter, add 30ml of water to the filtrate, and cool At about 5°C, adjust the pH to 1-2 with 10% hydrochloric acid, stir for 8 hours after dripping, filter with suction, wash with ice water, and vacuum-dry at 30°C to obtain 6.6 g of a light yellow solid with a yield of 87% and a purity of 95%;
[0052] The third step: 7β-[2-(2-formylamino...
Embodiment 3
[0055] Embodiment 3, the first step is with embodiment 1;
[0056] The second step: 7β-[2-(2-formylaminothiazol-4-yl)-2-(methoxyiminoacetamide)]-3-hydroxyl-3-cephem-4-carboxylic acid (III ) synthesis: 20 milliliters of N, N-dimethylformamide, 20 milliliters of water are charged into a 200 milliliter three-necked flask, 3 grams of compound (II) synthesized in the first step are added, and saturated sodium bicarbonate solution is added dropwise to pH= 7-8, cool to 0-5°C, add 4.0 grams of active ester in batches, control the pH=7-8 with saturated sodium bicarbonate solution while adding, slowly raise the temperature to about 50°C after the addition, stir for 4 hours, filter , add 30 ml of water to the filtrate, cool to about 5°C, adjust the pH to 1-2 with 10% hydrochloric acid, stir for 8 hours after dripping, filter with suction, wash with ice water, and dry in vacuum at 30°C to obtain 6.5 g of a light yellow solid. 86% yield, 95% purity;
[0057] The third step: 7β-[2-(2-form...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com