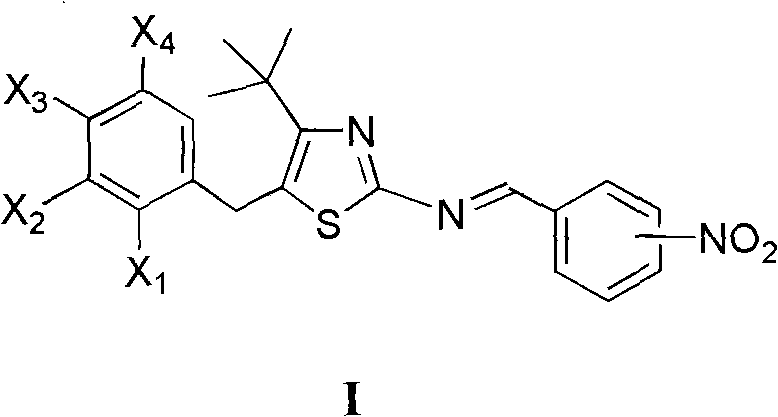
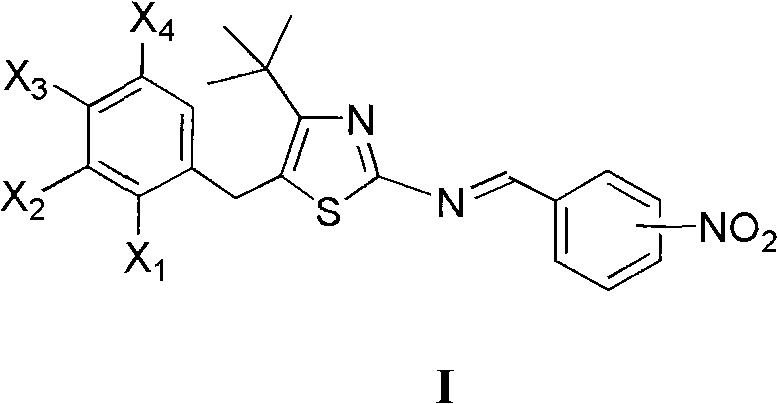
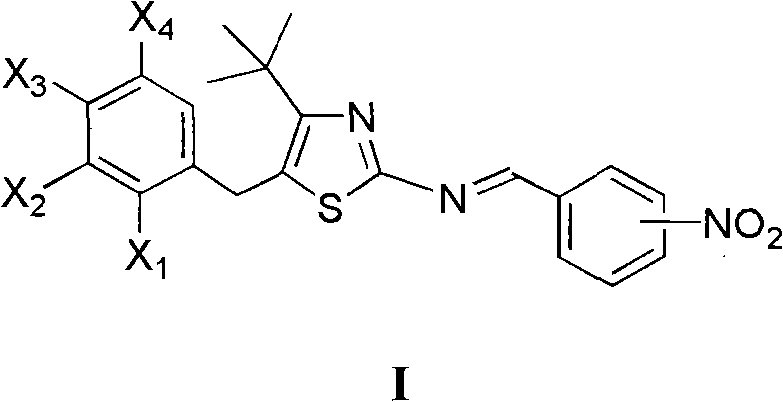
4-tertiary butyl-2-(nitrobenzyl imino) thiazole derivative as well as preparation method and application thereof
A nitrobenzylimino, tert-butyl technology, applied in the field of new compounds and their preparation, can solve the problems of the preparation of thiazole derivatives and the lack of research reports on antitumor activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 Preparation of 5-(2-chlorobenzyl)-4-tert-butyl-2-(3-nitrobenzimino)thiazole
[0015]
[0016] Dissolve 1.0mmol 5-(2-chlorobenzyl)-4-tert-butyl-2-aminothiazole in 10mL toluene, stir at room temperature, add dropwise 1.0mmol 3-nitrobenzaldehyde in toluene, add 0.2mL piperidine , Refluxed water separation reaction 2.0h. Cool, filter with suction, and recrystallize to obtain 5-(2-chlorobenzyl)-4-tert-butyl-2-(3-nitrobenzimino)thiazole, m.p.167~170°C, yield 62.4%, 1 H NMR (400MHz, CDCl 3 )δ: 1.47(s, 9H, 3×CH 3 ), 4.40 (s, 2H, CH 2 ), 7.18~7.42 (m, 4H, 2-ClC 6 h 4 ), 7.66(t, J=8.0Hz, J=8.0Hz, 1H, 3-O 2 NC 6 h 4 5-H), 8.29(d, J=8.0Hz, 1H, 3-O 2 NC 6 h 4 4-H), 8.34(ddd, J=8.0Hz, J=2.4Hz, J=2.0Hz, 1H, 3-O 2 N C 6 h 4 6-H), 8.77(t, J=2.0Hz, J=1.6Hz, 1H, 3-O 2 NC 6 h 4 2-H), 8.89 (s, 1H, N=CH).
Embodiment 2
[0017] Example 2 Preparation of 5-(4-chlorobenzyl)-4-tert-butyl-2-(3-nitrobenzylimino)thiazole
[0018]
[0019] Dissolve 1.0mmol 5-(4-chlorobenzyl)-4-tert-butyl 2-aminothiazole in 5mL benzene, add dropwise 1.0mmol 3-nitrobenzaldehyde in benzene solution, 0.2mL piperidine catalyzed, reflux for 2.5h . Cool, filter with suction, and recrystallize to obtain 5-(4-chlorobenzyl)-4-tert-butyl-2-(3-nitrobenzimino)thiazole, mp126~128°C, yield 76.3%, 1 H NMR (400MHz, CDCl 3 )δ: 1.46(s, 9H, 3×CH 3 ), 4.30 (s, 2H, CH 2 ), 7.16 (d, J=8.4Hz, 2H, 4-ClC 6 h 4 2,6-H), 7.31 (d, J = 8.4Hz, 2H, 4-ClC 6 h 4 3,5-H), 7.66 (t, J=8.0Hz, 1H, 3-O 2 NC 6 h 4 5-H), 8.29(d, J=7.6Hz, 1H, 3-O 2 NC 6 h 4 4-H), 8.35(dd, J=8.0Hz, J=1.2Hz, 1H, 3-O 2 NC 6 h 4 6-H), 8.78(d, J=1.6Hz, 1H, 3-O 2 NC 6 h 4 2-H), 8.90 (s, 1H, N=CH).
Embodiment 3
[0020] Example 3 Preparation of 5-(2,4-dichlorobenzyl)-4-tert-butyl-2-(3-nitrobenzimino)thiazole
[0021]
[0022] Dissolve 1.0mmol 5-(2,4-dichlorobenzyl)-4-tert-butyl-2-aminothiazole in 10mL toluene, stir at room temperature, add dropwise the toluene solution of 1.0mmol 3-nitrobenzaldehyde, add 0.2 mL piperidine, reflux and divide water for 2.5h. Cool, filter with suction, and recrystallize to obtain 5-(2,4-dichlorobenzyl)-4-tert-butyl-2-(3-nitrobenzimino)thiazole, m.p.171~174°C, yield 78.8% , 1 HNMR (400MHz, CDCl 3 )δ: 1.45(s, 9H, 3×CH 3 ), 4.37 (s, 2H, CH 2 ), 7.10 (d, J=8.4Hz, 1H, C 6 h 3 6-H), 7.23 (dd, J=8.4Hz, J=2.0Hz, 1H, C 6 h 3 5-H), 7.44(d, J=2.0Hz, 1H, C 6 h 3 3-H), 7.66(t, J=8.0Hz, J=8.0Hz, 1H, 3-O 2 NC 6 h 4 5-H), 8.29(d, J=8.0Hz, 1H, 3-O 2 NC 6 h 4 4-H), 8.33~8.38(m, 1H, 3-O 2 NC 6 h 4 6-H), 8.78(t, J=1.6Hz, J=1.6Hz, 1H, 3-O 2 NC 6 h 4 2-H), 8.92 (s, 1H, N=CH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com