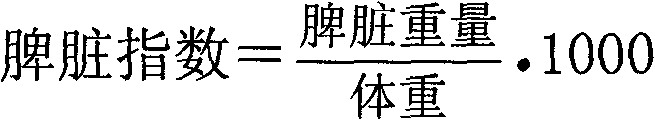Use of chlorogenic acid in preparing medicament for treating marrow fibrillation
A technology for bone marrow fibrosis and chlorogenic acid, which can be used in drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., and can solve problems such as chlorogenic acid that have not been discovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0035] [Experimental Example 1] The effect of chlorogenic acid on bone marrow suppression caused by physical factors in mice
[0036] Chlorogenic acid, with a content of 99.56%, is prepared into the required concentration with sodium chloride injection. Positive control drug: recombinant human granulocyte colony-stimulating factor injection, 300ug / bottle, 1.2ml / bottle.
[0037] SPF grade ICR mice, weighing 22-30 g, 240. According to body weight and sex, they were randomly divided into high-, medium-, and low-dose groups of chlorogenic acid, positive control group, model control group, and normal control group, with 40 rats in each group, half male and half male. Except for the normal control group, other animals were treated with 60 Whole body irradiation with Coγ-rays, the irradiation dose is 4Gy (dose rate 256Gy / h, irradiation time is 2min), after irradiation, each group is given the corresponding test substance according to Table 1 (the control group and the model group a...
experiment example 2
[0094] [Experimental Example 2] The effect of chlorogenic acid on bone marrow suppression caused by chemical factors in dogs
[0095] Chlorogenic acid, content 99.56%, positive control drug: Likejun tablet, 20mg / tablet, three times a day (3 tablets), cyclophosphamide for injection (abbreviated as CY), white powder, 200mg / ampule, 5 tubes / boxed. Beagle dogs, 36 (half male and half female), normal, healthy, uniform weight, non-pregnant female, weighing 6-7kg, aged 6 months.
[0096] See Table 19 for dose design.
[0097] Table 19 Dose Design
[0098]
[0099] Except the normal control group, the other 5 groups of dogs were intravenously injected with 8mg / ml cyclophosphamide 0.8ml / kg (8mg / kg), once a day for 5 consecutive days. On the 6th day, each test drug was administered according to Table 19, and normal saline was administered to the model control group and the normal control group for 13 consecutive days. Before modeling, every day after modeling, and 2, 4, 6, 8, 10,...
experiment example 3
[0127] [Experimental Example 3] Chlorogenic acid pair 60 Protective Effect of Co-γ-ray Injury on Spleen Hematopoietic Stem Cells in Mice
[0128] 1. Experimental method
[0129] Take Health C 57Sixty mice were randomly divided into 5 groups according to sex and body weight. Administration is divided into groups respectively, each mouse of negative group injects normal saline 0.4ml / 20g body weight intraperitoneally, and each mouse of positive group (granulocyte colony-stimulating factor) gives 0.4ml / 20g body weight (2ug / kg) by intraperitoneal injection, and all the other three groups are treated with 0.1%, 0.05% and 0.025% chlorogenic acid medicinal solution was intraperitoneally injected 0.4ml / 20g body weight (dose 20, 10 and 5mg / kg) to each mouse in each group. All rats in the above five groups were administered once a day for 7 consecutive days, and the body weight of the surviving animals was weighed on the 7th day, and 1 hour after the last administration. The mice in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com