Fusion protein of immunoglobulin fc and human apolipoprotein(a) kringle fragment
A technology of human immunoglobulin and fusion protein, which is applied in the field of fusion protein of immunoglobulin Fc and human apolipoprotein (a) Kringle fragment, which can solve the problems of prolonging half-life and reducing INF-α activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
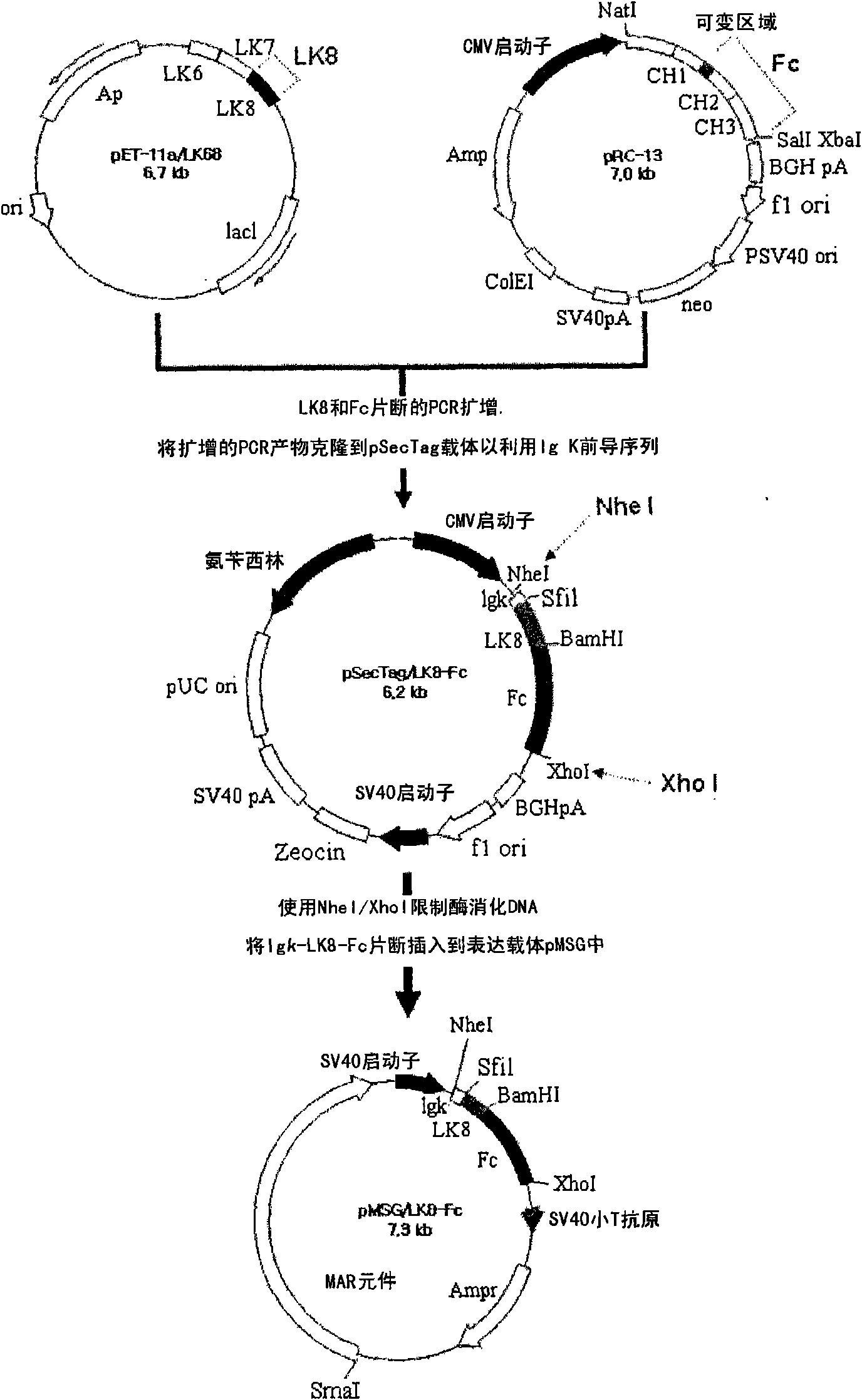
[0050] Example 1: Construction of recombinant vector expressing LK8-Fc fusion protein
[0051] To construct a vector encoding a fusion protein of LK8 and Fc, the LK8 gene (SEQ ID NO: 1) was obtained by PCR using the pET11B vector (WO2001 / 019868) containing the LK8 gene previously prepared by the inventors of the present invention as a template. In addition, the gene (SEQ ID NO: 2) encoding Fc was obtained by PCR using the pRC13-Hpa vector (Korean Patent 467706) as a template. The primers used in each PCR are shown in Table 1 below.
[0052] Specifically, PCR reactions were performed under the following conditions: template DNA was denatured at 94°C for 5 min, followed by 30 cycles of 30 sec at 94°C, 30 sec at 56°C, and 1 min at 72°C, followed by extension at 72°C. 5min. Also, for ease of cloning, a restriction enzyme digestion site was inserted into each primer so that the resulting PCR product had a restriction enzyme digestion site.
[0053]The two gene fragments genera...
Embodiment 2
[0058] Example 2: Establishment of an animal cell line expressing a large amount of LK8-Fc fusion protein
[0059] In order to establish an animal cell line producing the LK8-Fc fusion protein, the pMSG / LK8-Fc constructed in Example 1 was combined with the dihydrofolate reductase DHFR (dihydrofolate reductase) gene (Columbia University, USA) using Dosper (Roche, Switzerland). ) were transfected together into the cell line CHO DG44 (Columbia university, USA) in which the DHFR gene was deleted. Then, by this cell line, the bacterium colonies suitable for the MEM-α minimal medium containing 10% serum were initially selected, and by gradually increasing MTX (Methotrexate; ChoongWaePharma Corporation, Korea) concentration (including 50nM and 1μM) to Selected colonies were subcultured. During secondary culture, among the colonies showing resistance to MTX, a second selection was made for cell lines secreting high amounts of the protein of interest. The selected cell line was cul...
Embodiment 3
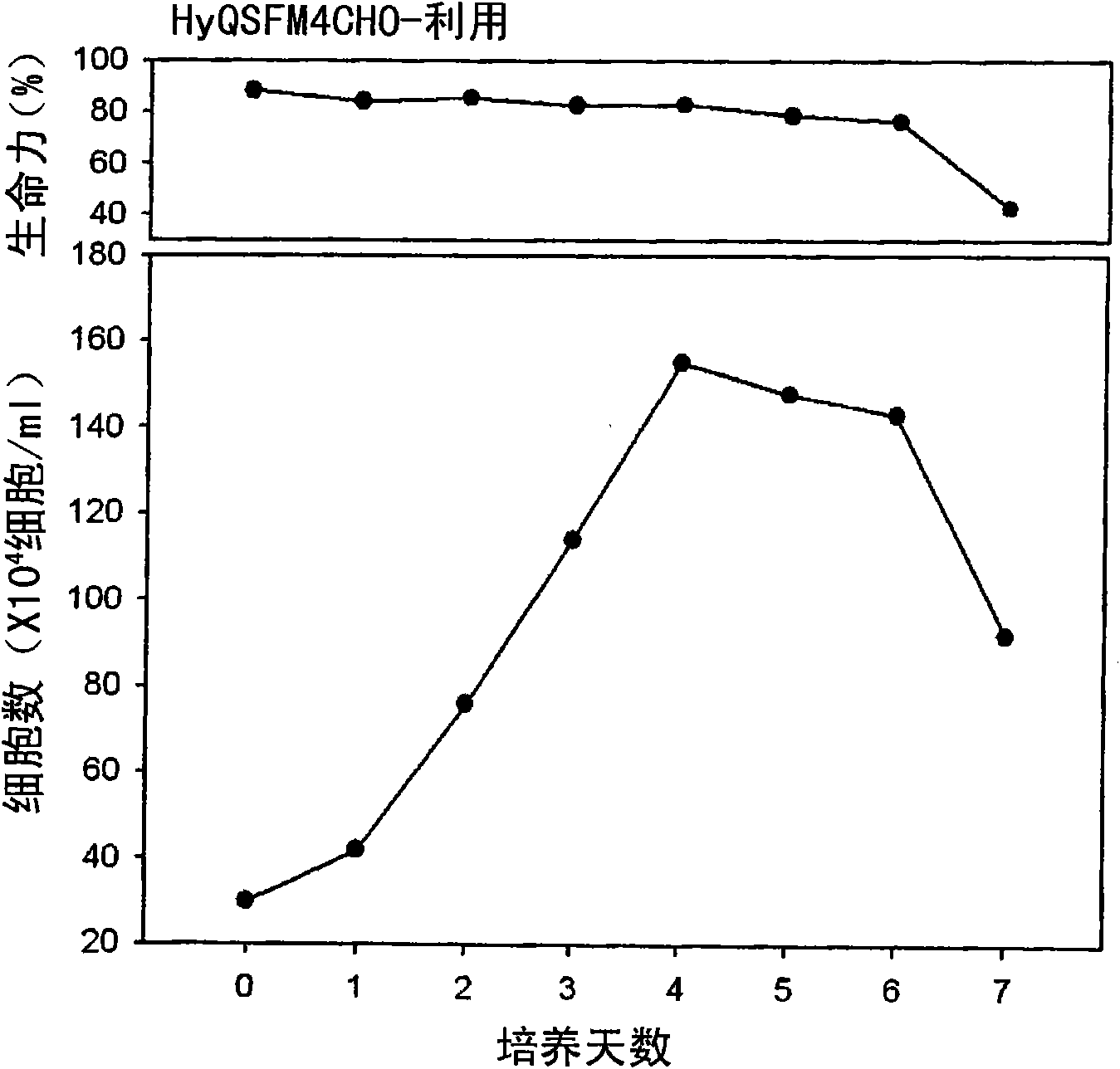
[0060] Embodiment 3: Purification of LK8-Fc fusion protein
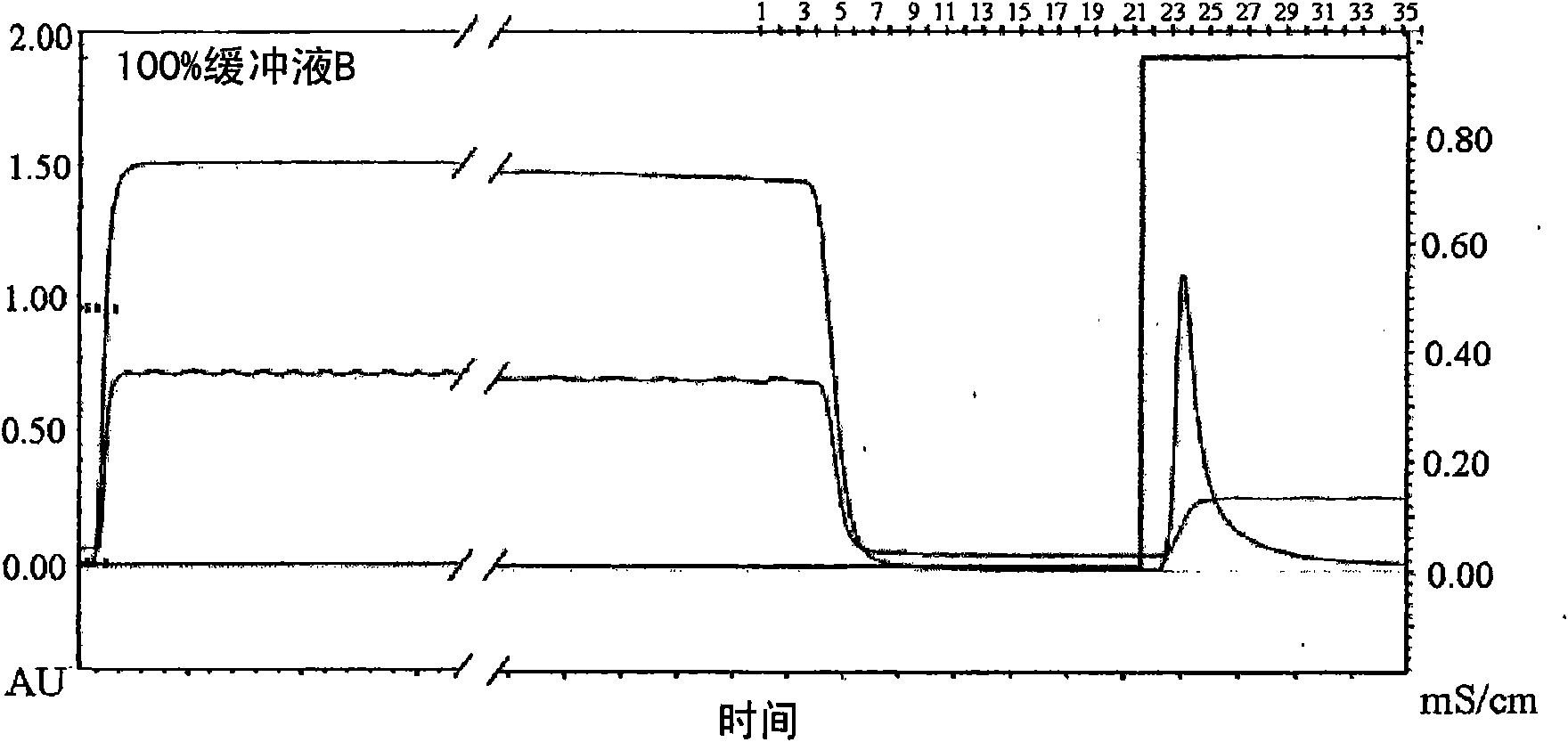
[0061] In order to purify the LK8-Fc fusion protein, the CHO / LK8-Fc cell line was rotationally cultured in the same manner as in Example 2 in the HyQ-SFM-CHO medium. Such as figure 2 shown. The cells were cultured and the growth and development ability of the cells were observed on the sixth day of culture, and the supernatant was collected by centrifugation. Then, the LK8-Fc fusion protein contained in the supernatant was purified in the following manner. On the basis of the fact that the Fc region of the LK8-Fc fusion protein has an affinity for protein G sepharose (Amersham Pharmacia, USA), affinity column chromatography was performed. Specifically, in a binding buffer (pH 6-8) containing 20-100 mM sodium phosphate, the LK8-Fc fusion protein contained in the supernatant was attached to a protein G sepharose column, and then used glycine buffer (pH 2-5) to elute it from the column ( image 3 ).
[0062] Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| half-life | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com