Method for producing anion exchange resin, anion exchange resin, method for producing cation exchange resin, cation exchange resin, mixed bed resin, and method for producing ultra-pure water for clean
A technology of cation exchange and exchange resin, applied in cation exchange materials, ion exchange water/sewage treatment, anion exchange, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0176] Specific methods for preparing the deoxidizing monomer include a method of bubbling the monomer with an inert gas, a method of membrane degassing, a method of circulating an inert gas to the upper gas phase of the monomer storage tank, and a column using a silica gel or the like. The method of processing. Alternatively, a commercially available deoxygenating monomer can also be used. Among them, the method of bubbling the monomer with an inert gas is preferable, and the inert gas used in this case is preferably nitrogen, carbon dioxide, or argon. In addition, deoxygenated monomers are stored in an inert gas atmosphere.
[0177] The amount of the deoxygenating monomer added is usually 10% by weight or more, preferably 50% by weight or more, more preferably 80% by weight or more, based on the total amount of the monomer mixture. When the amount of deoxygenated monomer added is too small, insufficiently polymerized oligomer components (dimers, trimers, oligomers), free p...
experiment example A
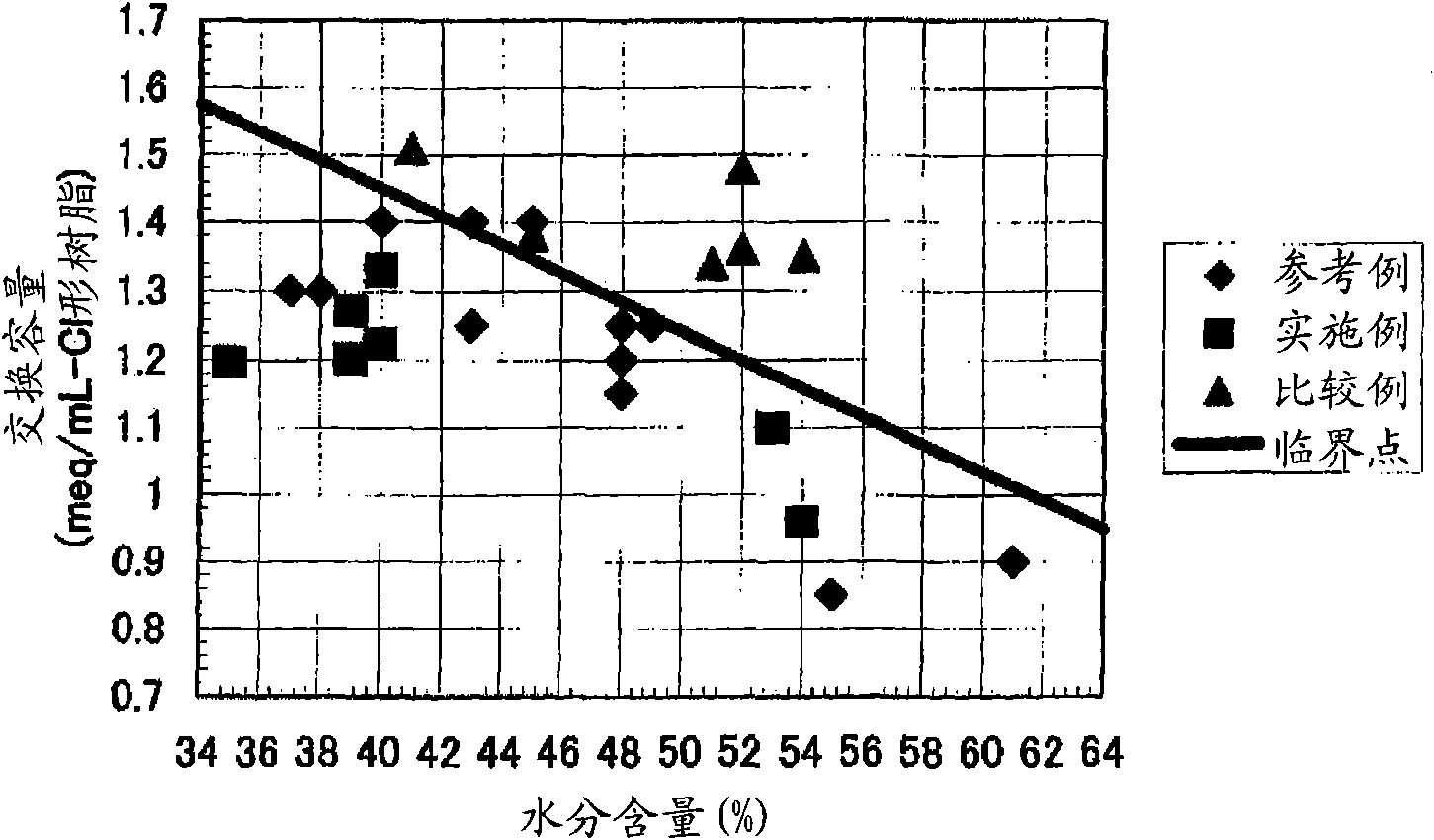
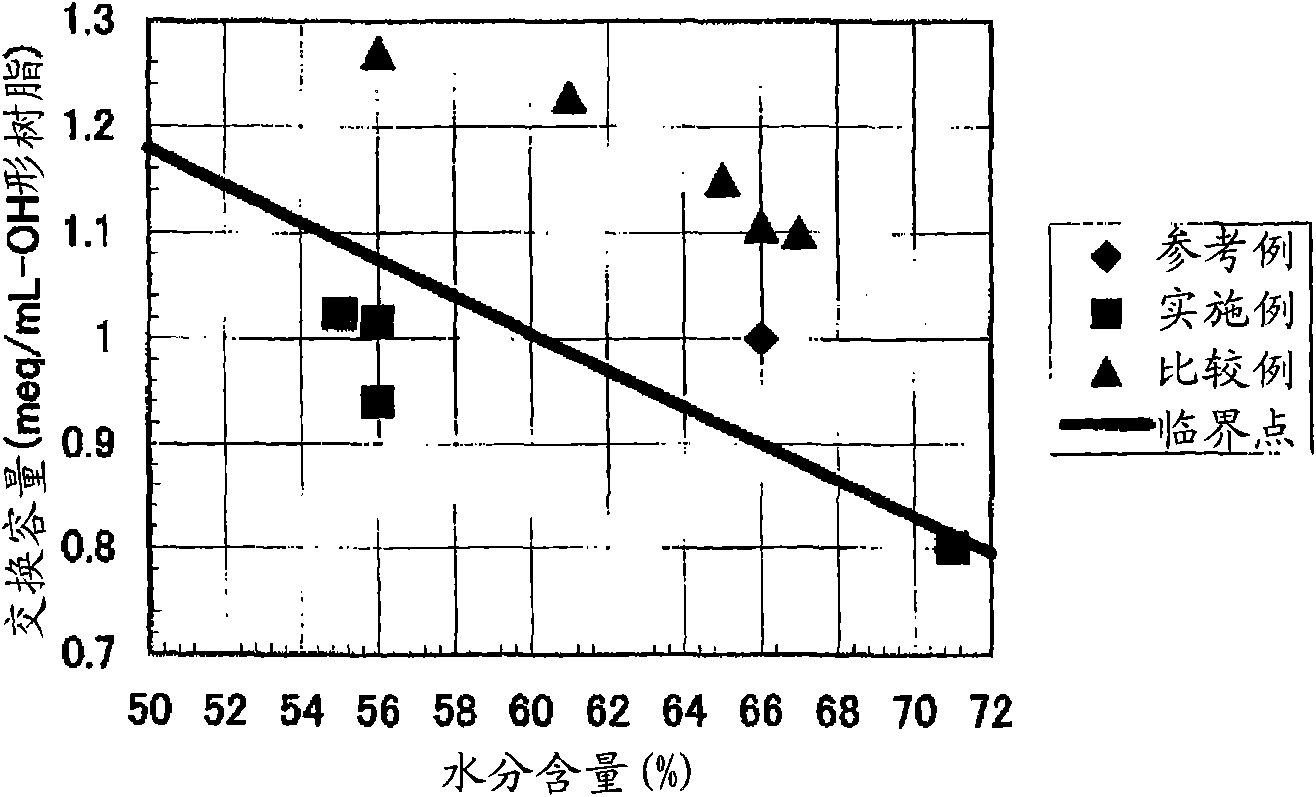
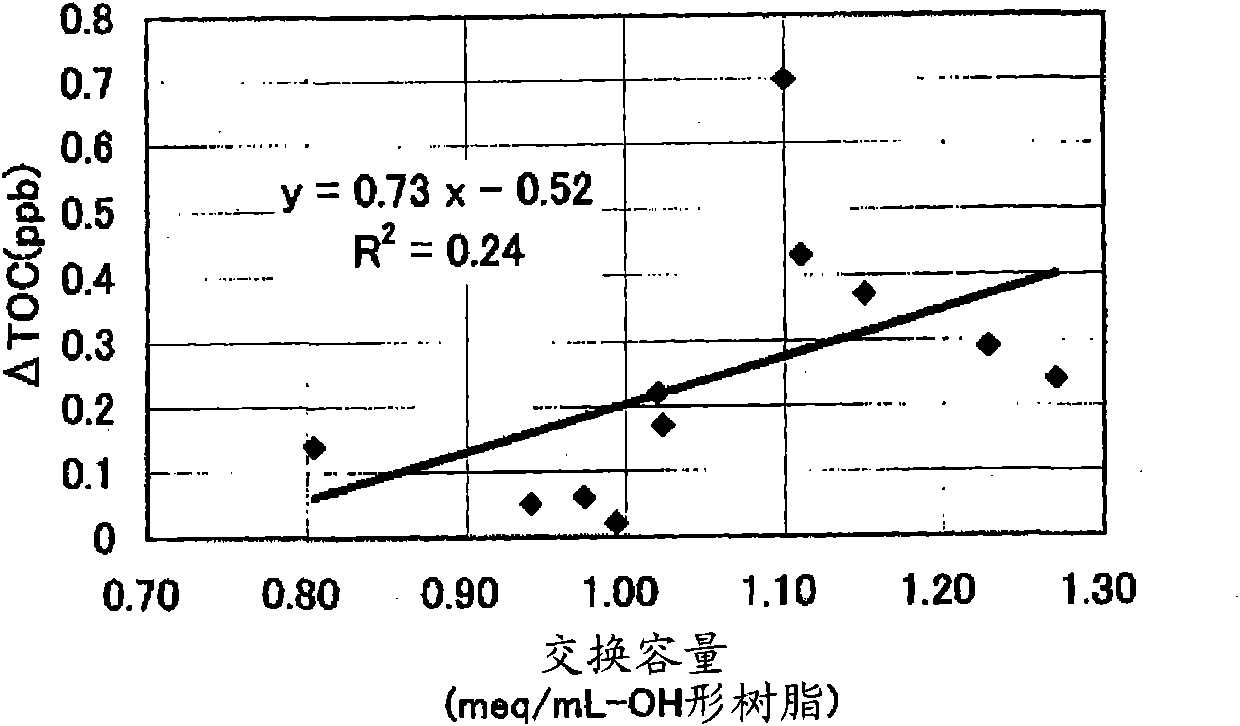
[0444] [Experimental example A: Experimental example showing the effect of the anion exchange resin of the first aspect of the present invention]
Embodiment 1
[0446] 590g of styrene (industrial grade, manufactured by Idemitsu Corporation) and 85g (8% by weight relative to the total monomer amount) of divinylbenzene (industrial grade, purity 63% by weight, non-polymerizable impurity content 0.09% by weight, Dow Co., Ltd.), and nitrogen gas was passed through the monomer mixture at 1 L / min for 1 hour to prepare a deoxygenated monomer mixture with an oxygen concentration of 1 mg / L. 1.8 g of dibenzoyl peroxide (purity 75% by weight, wet product, manufactured by NOF), 1.4 g of tert-butyl peroxybenzoate (purity 99% by weight, manufactured by NOF) were mixed with this mixture, and Suspended in 2025 g of 0.1% polyvinyl alcohol (for industrial use, manufactured by Nippon Synthetic Chemicals Co., Ltd., grade GH-20) aqueous solution. While stirring the suspension, it was kept at 80° C. for 5 hours, and then reacted at 120° C. for 4 hours to obtain a crosslinked copolymer.
[0447] The obtained crosslinked copolymer was subjected to a dissolut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com