Methods, compositions, and formulations for the treatment of thyroid eye disease
A technology of composition and compound, applied in the field of treating thyroid eye disease, composition and preparation, capable of solving problems such as diplopia and loss of vision
Inactive Publication Date: 2010-01-13
尼奥特蒂克斯公司
View PDF3 Cites 7 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
While some GO patients suffer only mild eye discomfort, 3-5% suffer from severe pain and inflammation with diplopia and even vision loss
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 4
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Login to View More
Abstract
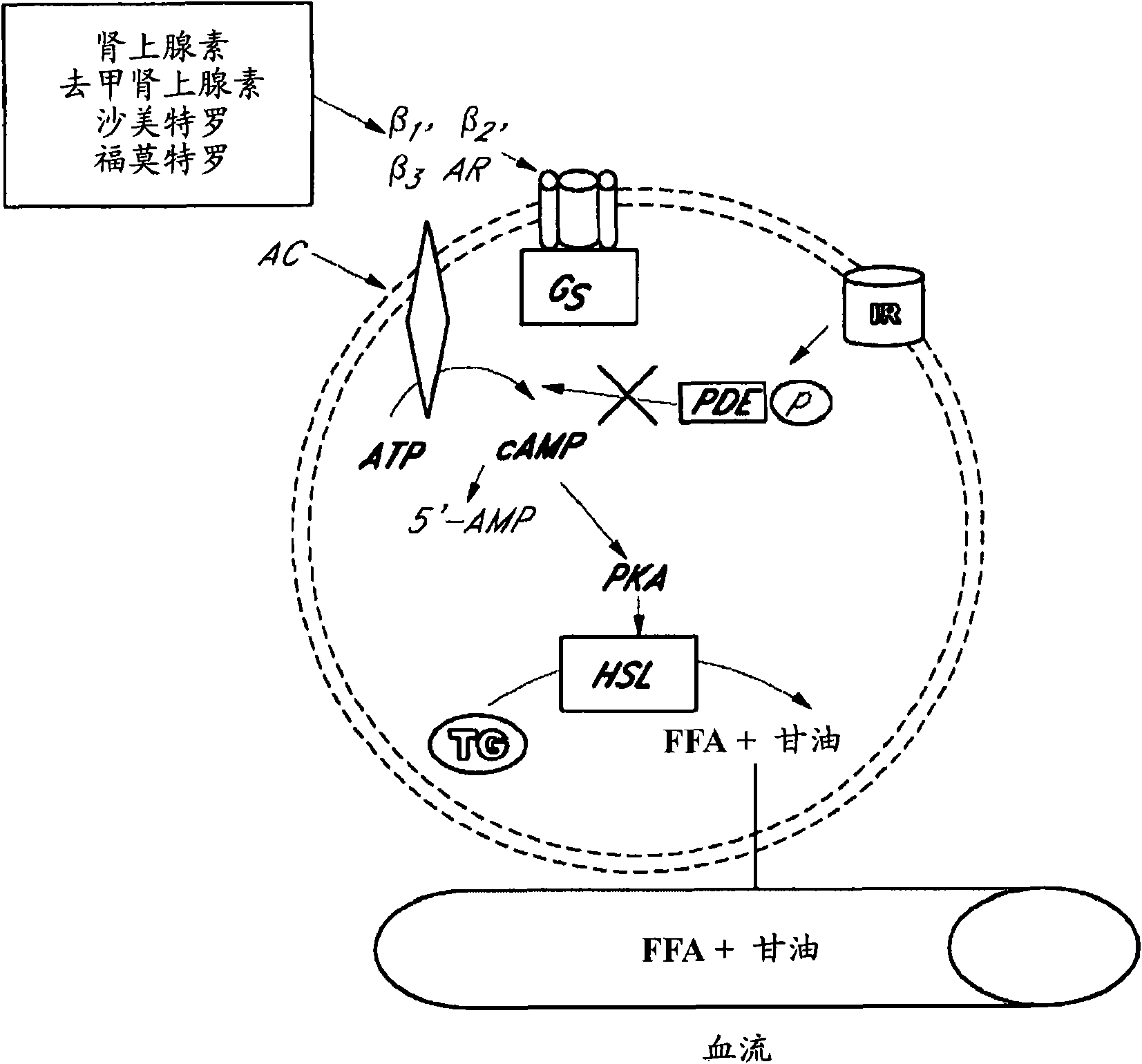
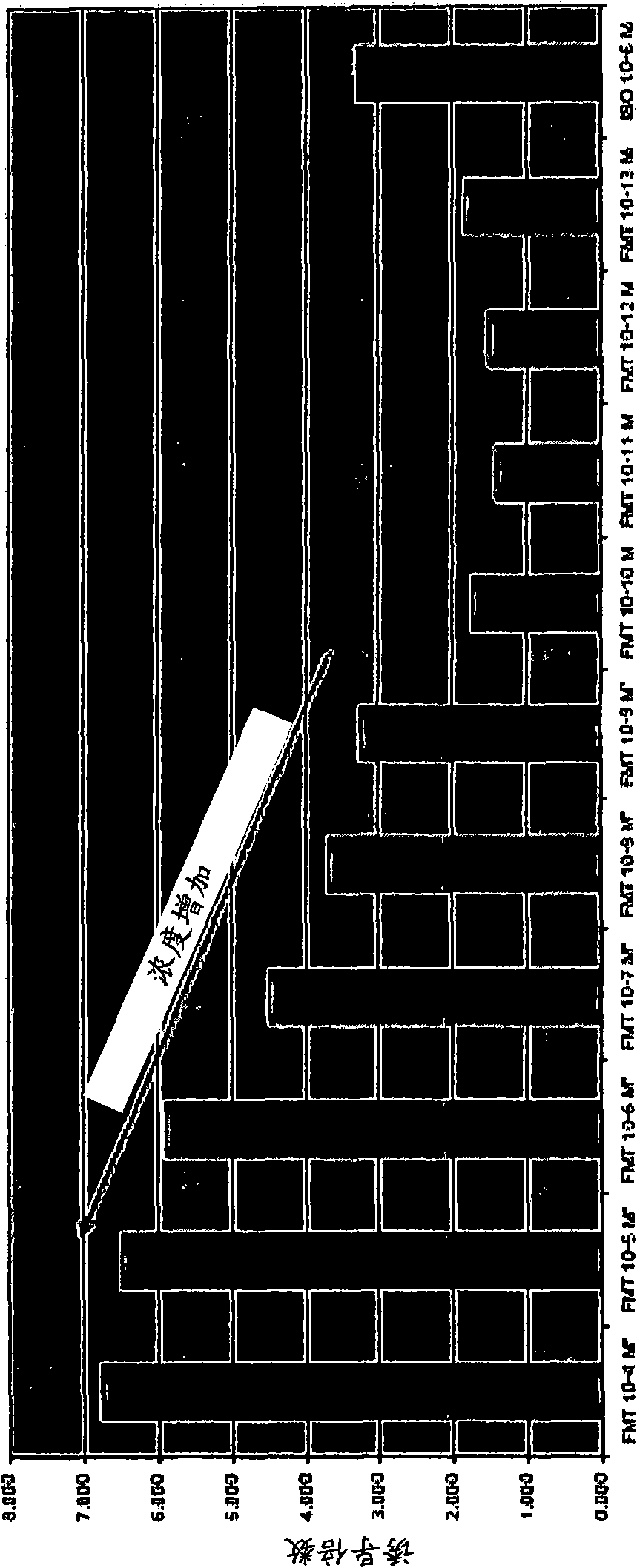
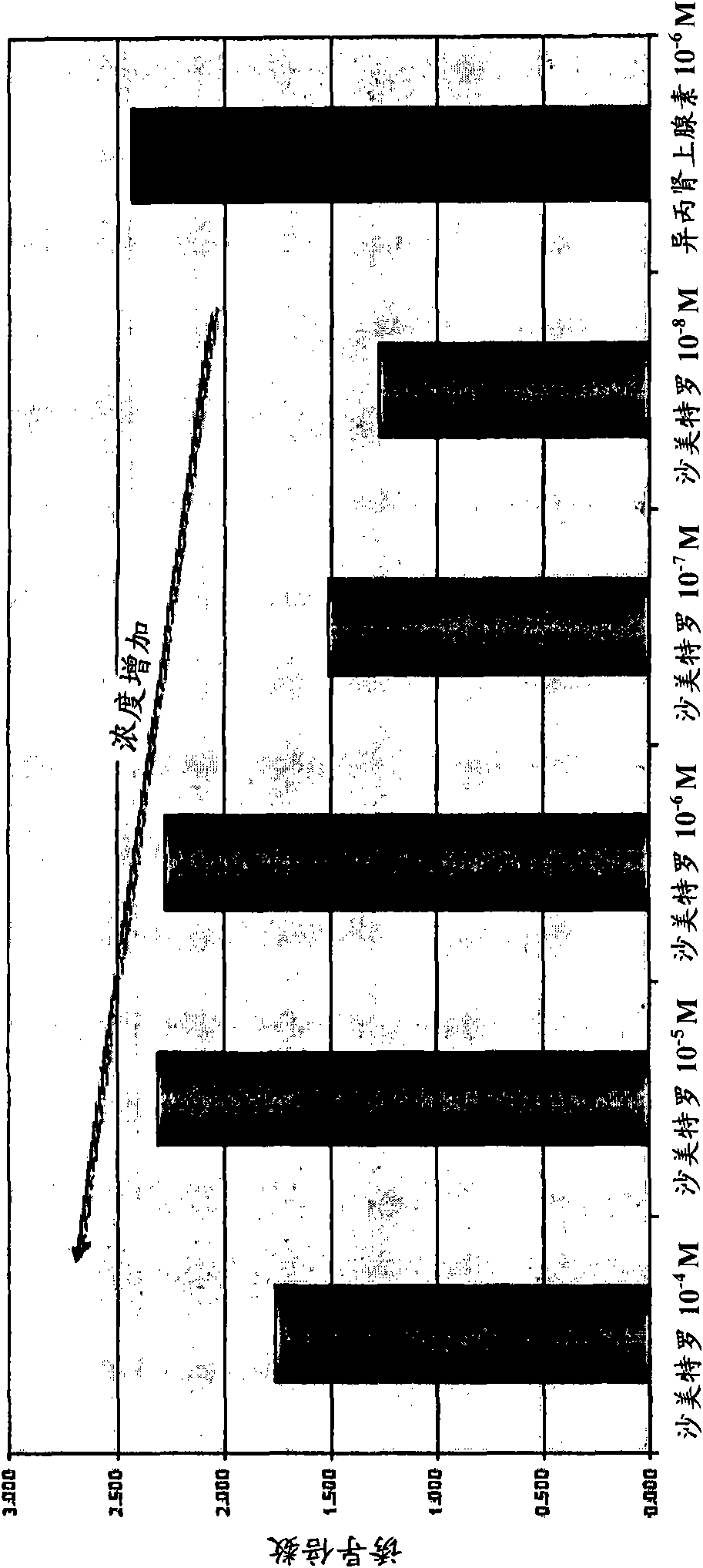
Compositions, formulations, methods, and systems for treating thyroid eye disease and related conditions (e.g., Grave's Ophthalmopathy). The methods described herein include administering, to a patient in need, systemic or local beta adrenergic agonists (e.g., as an extended release crystalline microparticle suspension). The methods can further include administering a compound for reducing beta adrenergic receptor desensitization (e.g., a corticosteroid) prior to administering or coadministered with the beta adrenergic agonist. The methods can also include locally administering to the eye an immunosuppressant agent (e.g., rapamycin) prior to administering a beta adrenergic agonist. The compositions described herein include ophthalmic pharmaceutical formulations of beta adrenergic agonists in the form of extended release crystalline microparticle suspensions or mixtures of the crystalline microparticle suspensions with beta adrenergic agonist solutions. The compositions also include ophthalmic formulations of a compound for reducing beta adrenergic receptor desensitization in the form of extended release crystalline microparticle suspensions.
Description
[0001] cross reference [0001] This application claims the benefit of U.S. Provisional Patent Application Serial Nos. 60 / 852,221, filed October 17, 2006, 60 / 898,009, filed January 29, 2007, and 60 / 919,011, filed April 13, 2007, which Incorporated herein by reference in its entirety. Background of the invention [0002] Graves' disease is a common disease with an incidence rate of 1 / 1000 women per year. In addition to hyperthyroidism, 25-50% of individuals with Graves' disease progress to clinically involving the eye, ie, thyroid eye disease. Graves' Ophthalmopathy (GO) is a typical form of thyroid eye disease. While some GO patients suffer only mild ocular discomfort, 3-5% suffer from severe pain and inflammation with diplopia and even vision loss. [0003] The clinical symptoms and signs of GO can be explained mechanistically as a marked increase in tissue volume within the bony orbit. Enlargement of the orbital tissue causes the globe to move forward, and outflow from t...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/135A61K31/167A61K31/575A61P3/04
Inventor J·D·多巴克
Owner 尼奥特蒂克斯公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com