Specific therapy and medicament using integrin ligands for treating cancer
一种整联蛋白、配体的技术,应用在药物组合、肽/蛋白质成分、含有效成分的医用配制品等方向,能够解决效力降低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
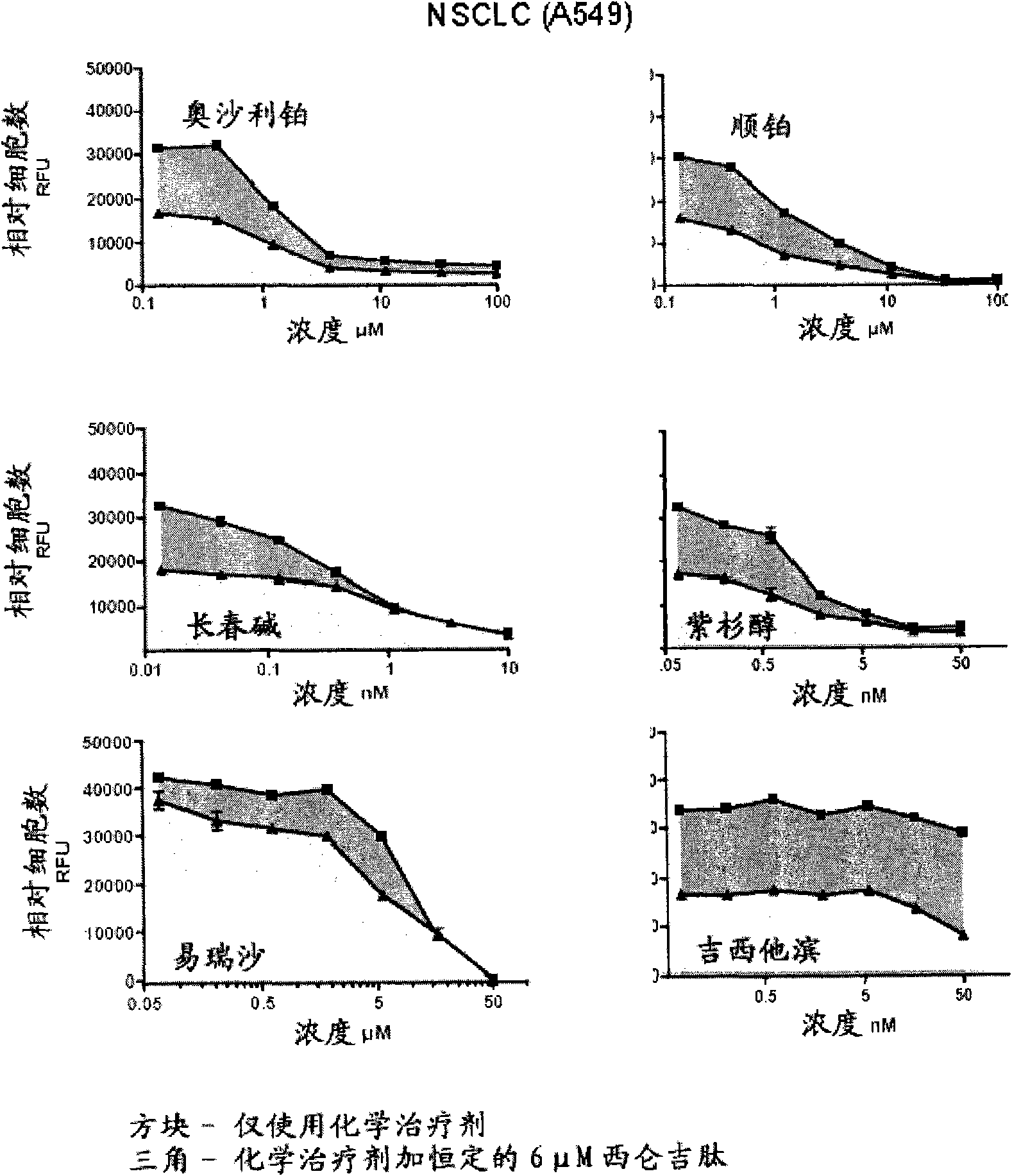
Examples
Embodiment 1
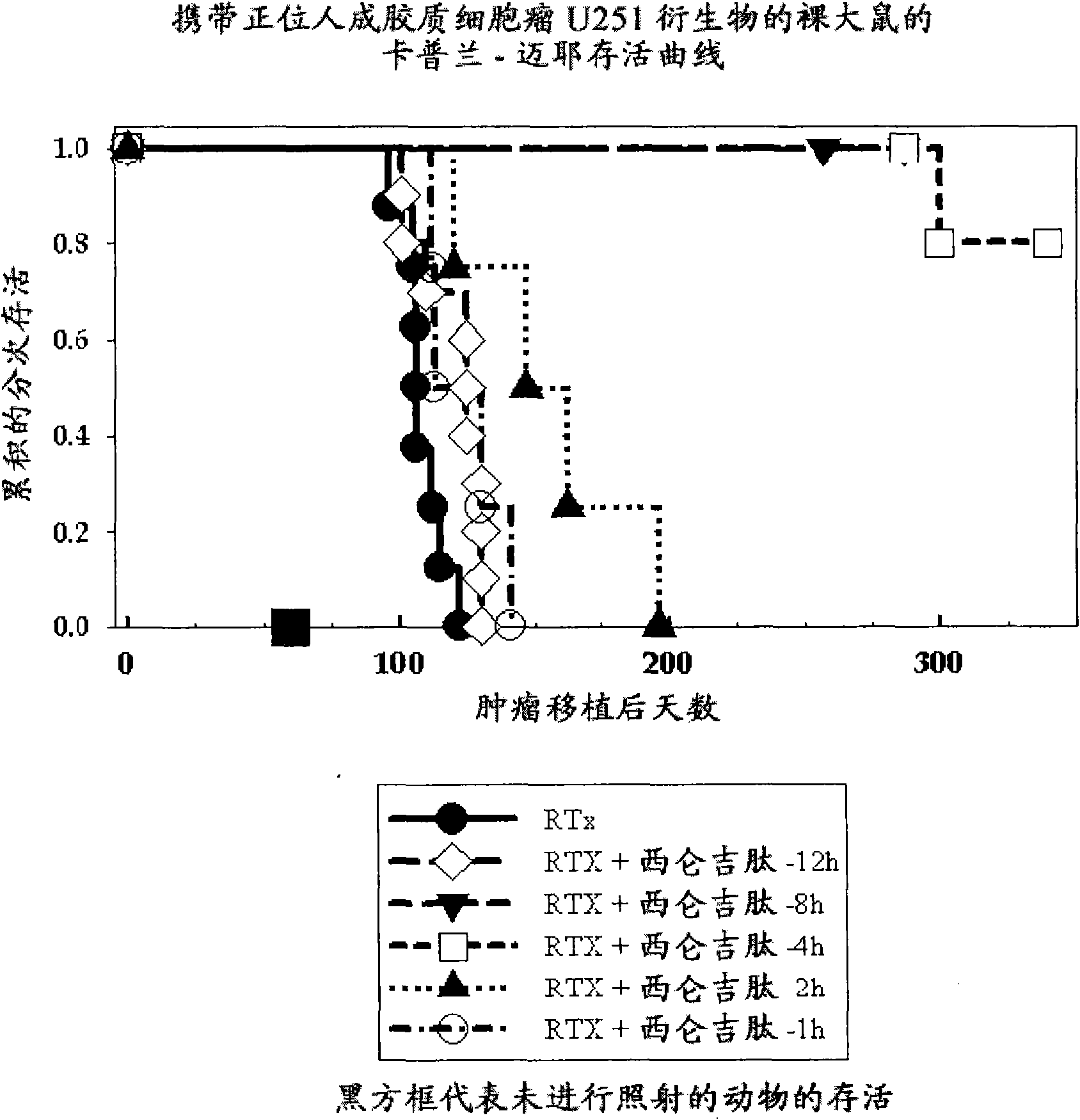
[0910] Example 1: Radiation therapy of a rat orthotopic glioblastoma model, cilengitide (=cyclo-(Arg-Gly-Asp-DPhe-NMe-Val)) schedule experiment
[0911] NIH rnu nude rats were anesthetized, restrained, and 5x10E5 U251 human glioblastoma suspended in 10 ul of medium was placed 1 mm retroorbitally and 3 mm to the right of the anterior blotch using a #2701 Hamilton syringe fitted with a 26 gauge needle , 2.5 mm deep intracerebral injections were performed essentially as previously published (Engebraaten et al., 1999). Fourteen days later, at various times (8 h, 8 h, 4h, 2h, 1h) Cilengitide (4 mg / kg) in PBS was administered as an intraperitoneal bolus. Animals also received the same intraperitoneal bolus of cilengitide each day for the next 7 days. Animals were maintained on an unrestricted diet until they were moribund, or for sampling for tissue analysis (in the t-4 and t-8 groups, the animals were still healthy 230 days after tumor injection). Kaplan-Meier survival curves we...
Embodiment 2
[0925] Example 2: Phase IIa trial of cilengitide ((=cyclo-(Arg-Gly-Asp-DPhe-NMe-Val)) single agent therapy in recurrent glioblastoma patients
[0926] Background: This phase IIa study was designed to evaluate integrin alpha as a single agent v beta 3 and alpha v beta 5 The inhibitor of the cyclic RGD pentapeptide cilengitide ((=cyclo-(Arg-Gly-Asp-DPhe-NMe-Val)) at 500 and 2000 mg doses in patients with recurrent glioblastoma (GBM) Safety, toxicity and clinical activity in (pts).
[0927] Methods: In this multicentre, open-label, randomized, uncontrolled study, patients with GBM and predictable disease (relapsed after prior treatment with temozolomide and radiation therapy) were randomized to receive twice-weekly intravenous doses of Cilengitide 500mg or 2000mg until progression. Histopathological diagnosis and magnetic resonance (MRI) imaging were used for independent blinded review. The primary endpoint was progression-free survival (PFS) at 6 months (mths). Secondary ...
Embodiment 3
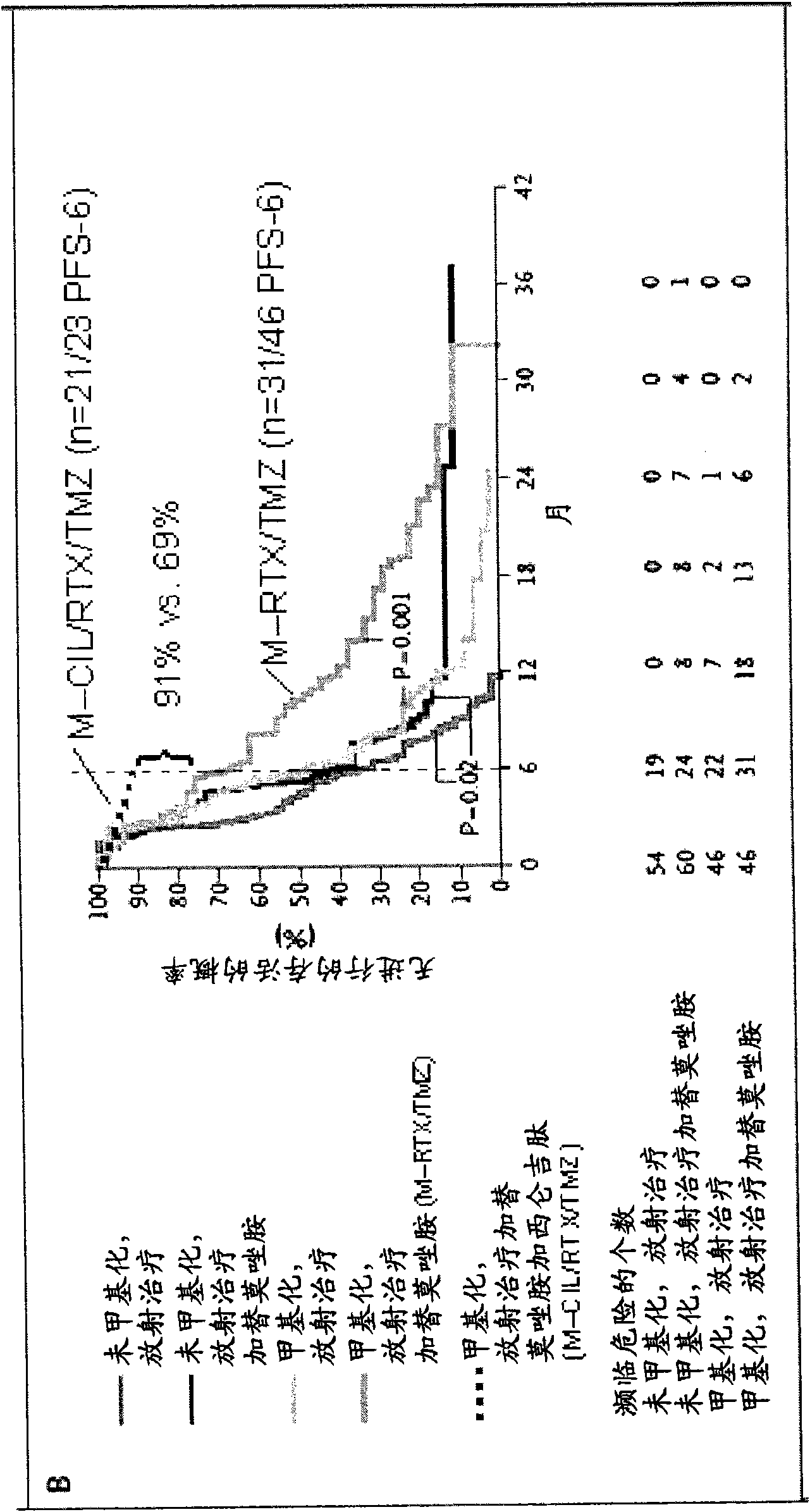
[0930] Example 3: Use of Cilengitide (= Cyclo-(Arg-Gly-Asp-DPhe-NMe-Val)) and Temozolomide and Simultaneously in Patients with Newly Diagnosed Glioblastoma (GBM) Radiation therapy followed by a phase I / IIa trial of maintenance temozolomide and cilengitide
[0931] Objective: To evaluate the combination of inhibitors of integrins avβ3 and avβ5 in combination with the cyclic RGD pentapeptide-cilengitide (=cyclic-(Arg-Gly-Asp-(Arg-Gly-Asp- DPhe-NMe-Val),) safety, toxicity and efficacy.
[0932] Patients and methods: 52 patients (PS 0-1: 92%, 2: 8%; age Median age 57) (Stupp et al NEJM 2005). In addition, cilengitide injections (500 mg twice weekly intravenously) were initiated one week prior to TMZ / RT and administered throughout chemotherapy or until progression. The primary endpoint was progression-free survival at 6 months (target: 65%). Patients then underwent MRI every 2 months. Looking at histopathological diagnosis and MRI imaging alone, the methylation status of the M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com