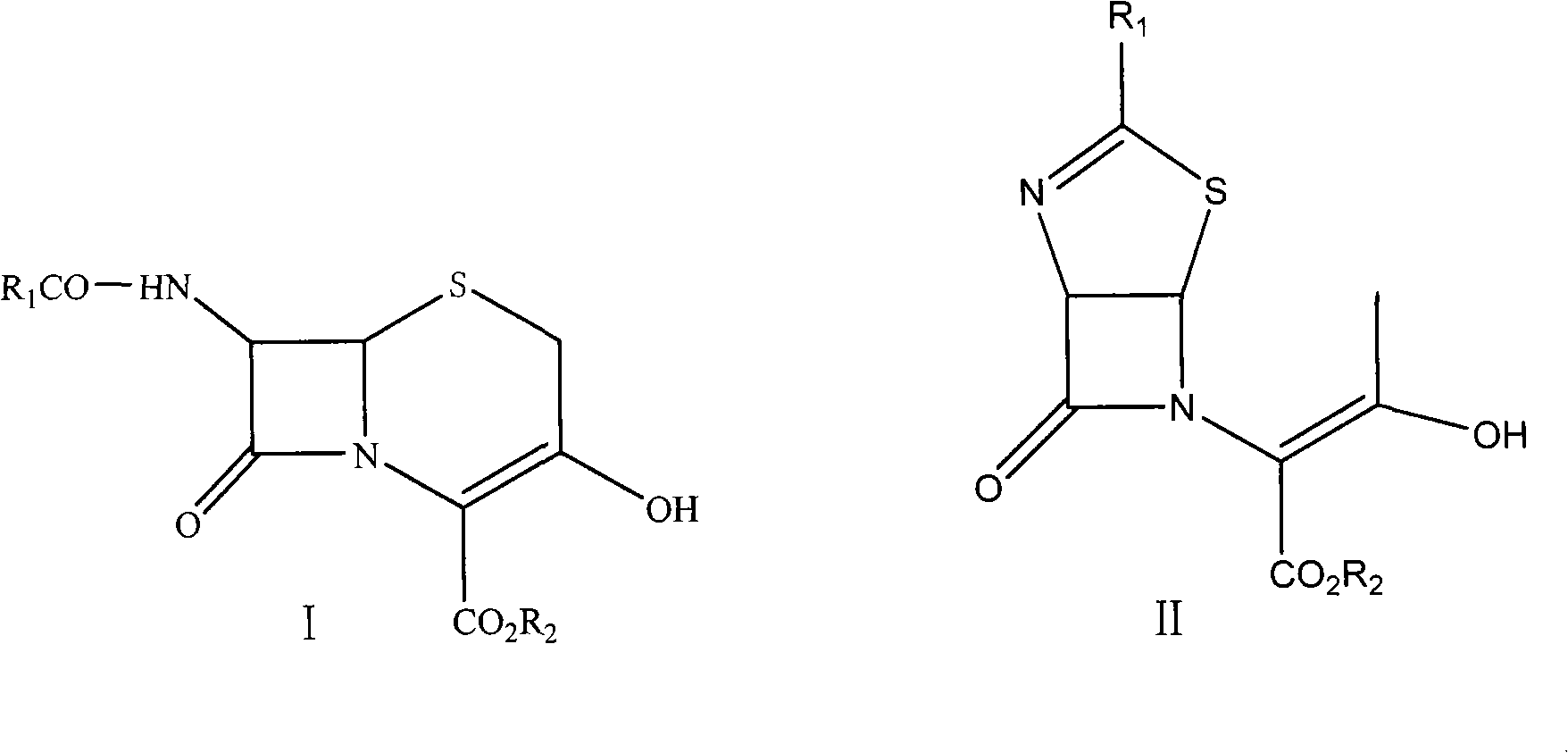
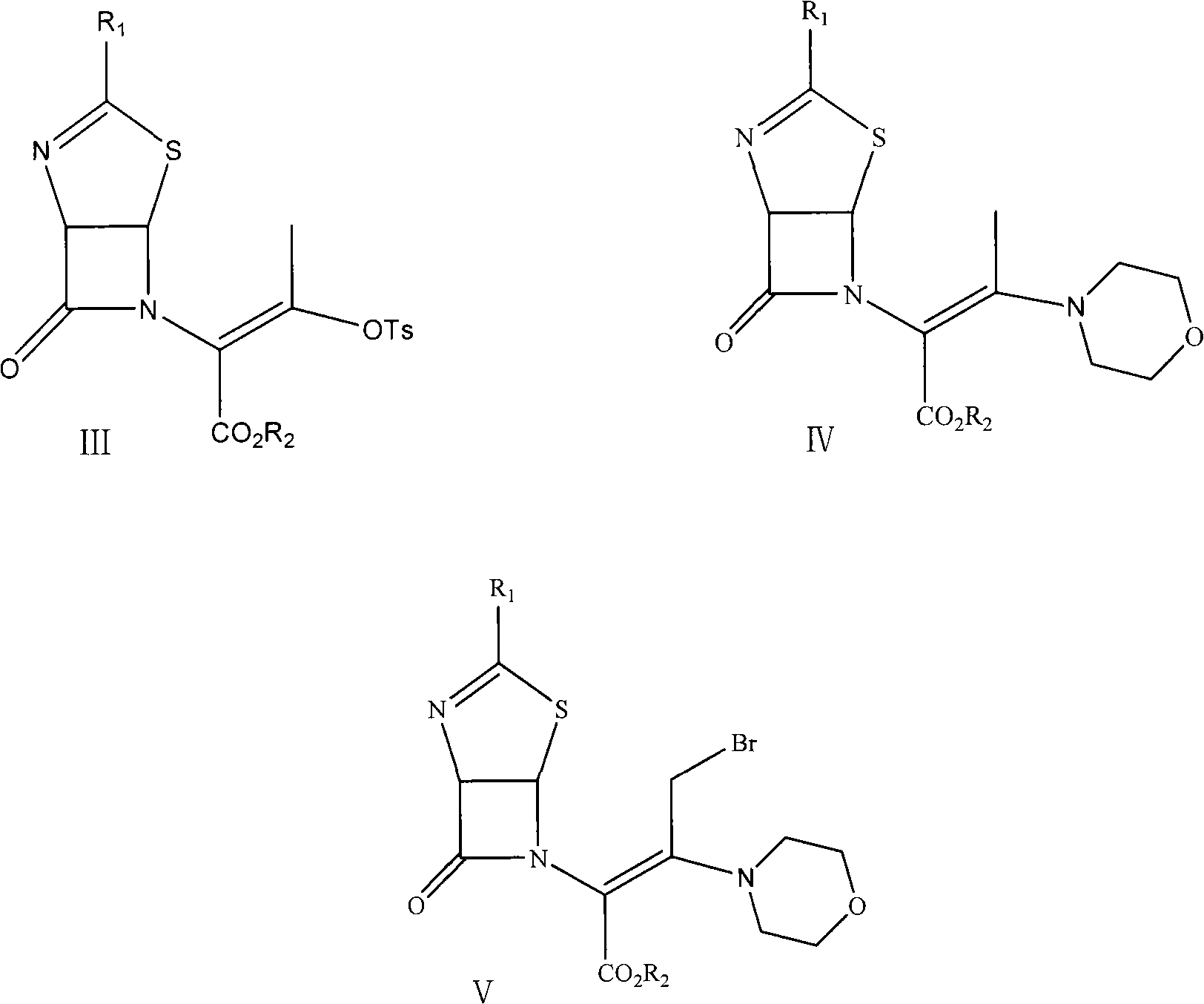
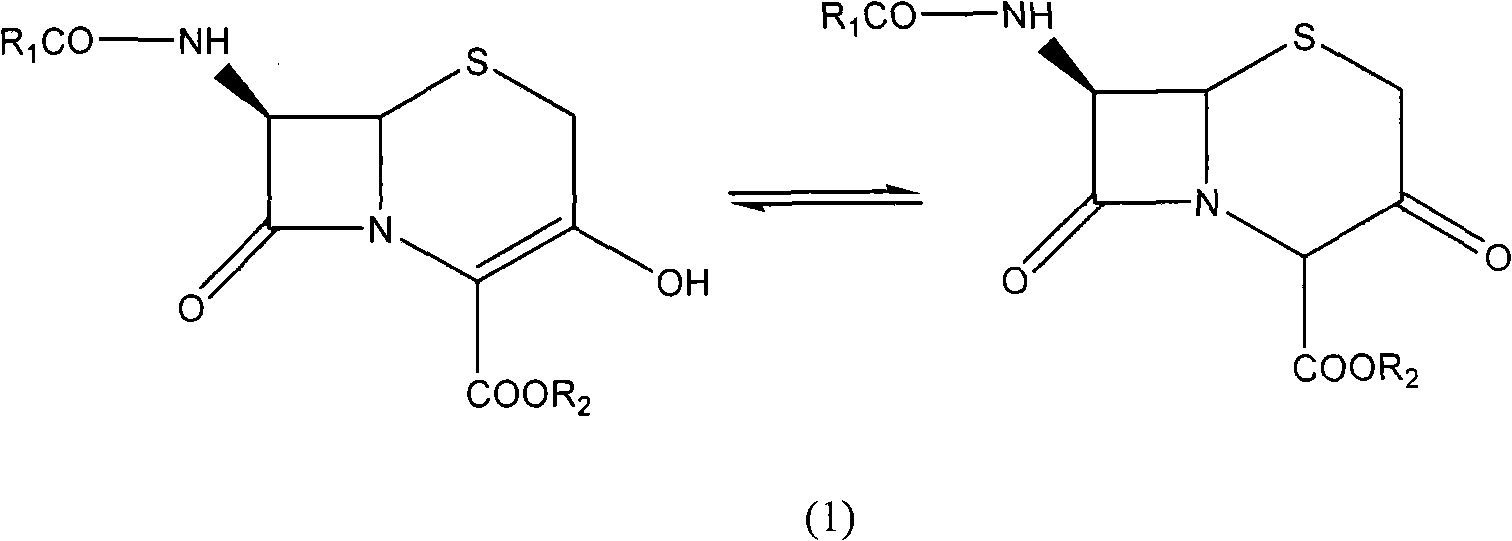
Preparation method of 3-hydroxy-cepham compound
A compound, cephalosporin technology, applied in the field of synthesis of pharmaceutical intermediates, can solve problems such as high cost, pollution, and low yield, and achieve the effects of low cost, safe operation, and low toxicity of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 2000ml dichloromethane and 300g structural formula in reaction bottle and be the compound thiazoline enol ester derivative of formula II (wherein R 1 for PhCH 2 CO group; R 2(p-nitrobenzyl) and p-toluenesulfonyl chloride 138.7g, stirred and dissolved, cooled to 0-5°C in ice water. Dilute ammonia water was slowly added dropwise to carry out sulfonylation reaction to obtain p-toluenesulfonate, a compound of formula III. After the reaction, a sample was taken to detect that the content of the compound of formula II in the reaction solution was less than 0.1%. The aqueous layer was removed, and dried by adding anhydrous magnesium sulfate. The temperature was lowered to 0° C. to -10° C., 57.6 g of morpholine was added, and then triethylamine was added dropwise to carry out enamination reaction to obtain a compound of formula IV. After the enamination reaction, the residual p-toluenesulfonate of the compound of formula III was monitored by HPLC to be less than 0.1%. C...
Embodiment 2
[0036] Add 2000ml tetrahydrofuran and 300g structural formula in reaction bottle and be the thiazoline enol ester derivative of formula II (wherein R 1 for PhCH 2 CO group; R 2 (p-nitrobenzyl) and p-toluenesulfonyl chloride 138.7g, stirred and dissolved, cooled to 5-10°C in ice water. Ammonia gas is slowly introduced to carry out sulfonylation reaction to obtain p-toluenesulfonate, a compound of formula III, and after the reaction, a sample is taken to detect that the content of the compound of formula II is less than 0.1%. The solvent tetrahydrofuran was removed under reduced pressure, and 1500 ml of dichloromethane and anhydrous magnesium sulfate were added to dry it. The temperature was lowered to -10°C to -20°C, 57.6g of morpholine was added, and then triethylamine was added dropwise to carry out enamination reaction to obtain the compound of formula IV. After the enamination reaction, the residual tosylate of the compound of formula III was monitored by HPLC to be less...
Embodiment 3
[0038] Add 2000ml dichloromethane and 300g structural formula in reaction bottle and be the thiazoline enol ester derivative of formula II (wherein R 1 for PhCH 2 CO group; R 2 (p-nitrobenzyl) and p-toluenesulfonyl chloride 138.7g, stirred and dissolved, cooled to 5-10°C in ice water. Dilute ammonia water was slowly added dropwise to carry out sulfonylation reaction to obtain the compound p-toluenesulfonate with the structural formula III. After the reaction, a sample was taken to detect that the content of the compound of the formula II was less than 0.1%. The aqueous layer was removed, and dried by adding anhydrous magnesium sulfate. Cool down to -20°C to -35°C, add 57.6g of morpholine, and then pass through ammonia gas to carry out enamination reaction to obtain the compound of formula IV. After the reaction was completed, the remaining p-toluenesulfonate of the compound of formula III was monitored by HPLC to be less than 0.1%. Continue to lower the temperature to -45°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com