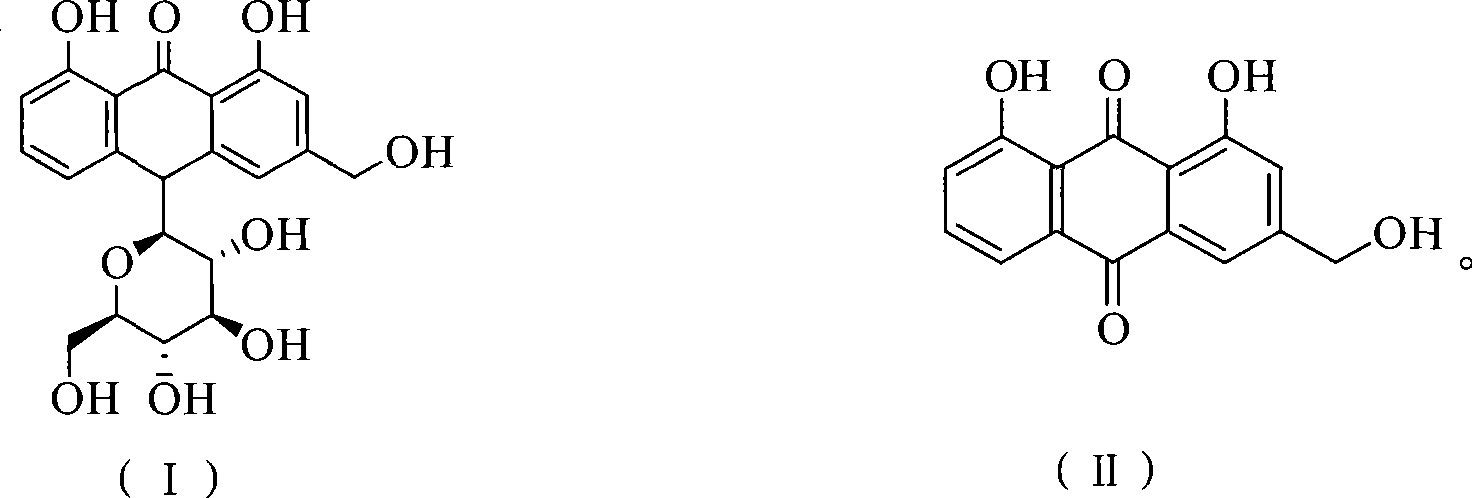
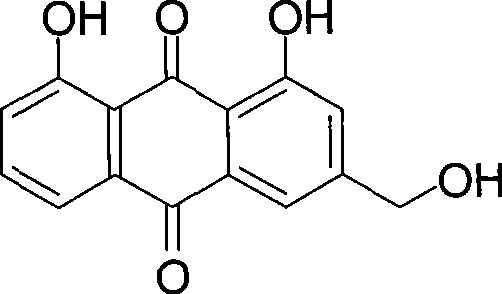
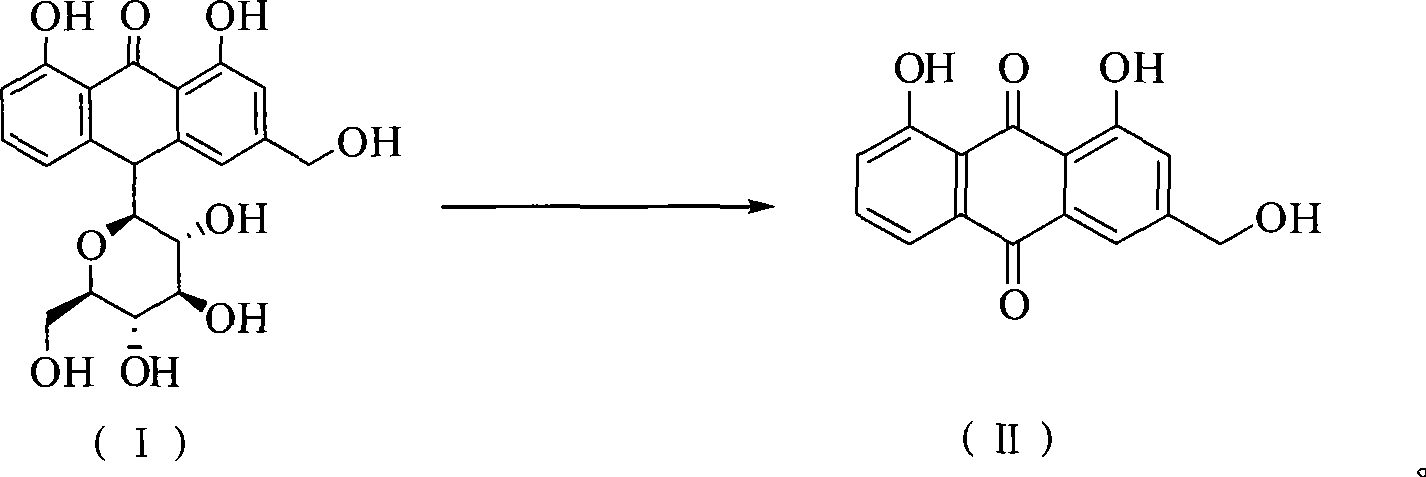
Method of preparing aloe-emodin
A technology of aloe-emodin and aloin, applied in the preparation of quinone oxide, organic chemistry, etc., can solve the problems of high pipeline design requirements, iron residue in products, increased risk, etc., and achieve good promotion and application prospects, high yield and purity , the effect of less solvent consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Put aloin (9.36g, 20mmol) and Oxone (24.59g, 40mmol) into a 150ml reaction vessel; add 20g water and 48g toluene; stir and react at 110°C for 2h. After the reaction is completed, filter while it is hot, and the filtrate is allowed to stand for layers. Take the toluene layer, dry it with anhydrous sodium sulfate, and recover part of the solvent by distillation. The residue is cooled to 0°C to crystallize, filter, and the filter cake is dried to obtain aloe-emodin 5.03g. The HPLC purity was 97.4%, and the yield was 90.7%.
Embodiment 2
[0025] Put aloin (9.36g, 20mmol) and Oxone (24.59g, 40mmol) into a 500ml reaction vessel; add 140g water and 48g toluene; stir and react at 110°C for 2h. After the reaction is completed, filter while it is hot, and the filtrate is allowed to stand for layers. Take the toluene layer, dry it with anhydrous sodium sulfate, and recover part of the solvent by distillation. The residue is cooled to 0°C to crystallize, filter, and the filter cake is dried to obtain aloe-emodin 5.18g. The HPLC purity was 96.2%, and the yield was 92.3%.
Embodiment 3
[0027] Put aloin (9.36g, 20mmol) and Oxone (24.59g, 40mmol) into a 250ml reaction vessel; add 75g water and 20g toluene; stir and react at 110°C for 2h. After the reaction is finished, filter while it is hot, let the filtrate stand to separate layers, take the toluene layer, dry it with anhydrous sodium sulfate, recover part of the solvent by distillation, cool the residue to 0°C to crystallize, filter, and dry the filter cake in vacuum to obtain aloe-emodin 5.16 g. The HPLC purity was 95.7%, and the yield was 91.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com