Methods for prediction and prognosis of cancer, and monitoring cancer therapy
A therapeutic and prognostic technology, applied in the field of biomarkers to monitor the effect of cancer treatment, can solve problems such as unreported correlations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1. Solid Phase Sandwich Microtiter ELISA of Human Serum and Plasma Sample Preparations
[0089] [083] Samples suitable for VEGF-165 ELISA analysis include human plasma treated with sodium heparin, citrate, or EDTA, as well as human serum. Special care must be taken in the preparation and assay of human serum and plasma due to possible interfering factors. Any flocculants should be removed from the samples by microcentrifugation prior to dilution. Serum or plasma samples for testing should have an initial concentration of approximately 12-13% (sample diluted 1:8 with sample diluent). For example, 40 μl of sample can be diluted into 280 μl of sample diluent and 100 μl added to each well of a microtiter plate.
[0090] Steps
[0091] [084] The following ELISA protocol was used in a sandwich ELISA (Oncogene Science, Cambridge, MA) to measure human VEGF-164 in human plasma or serum.
[0092] 1. Prepare the working solution (1X) for washing the plate (supplied as ...
Embodiment 2
[0110] Example 2. Plasma collected from patients with non-small cell lung cancer
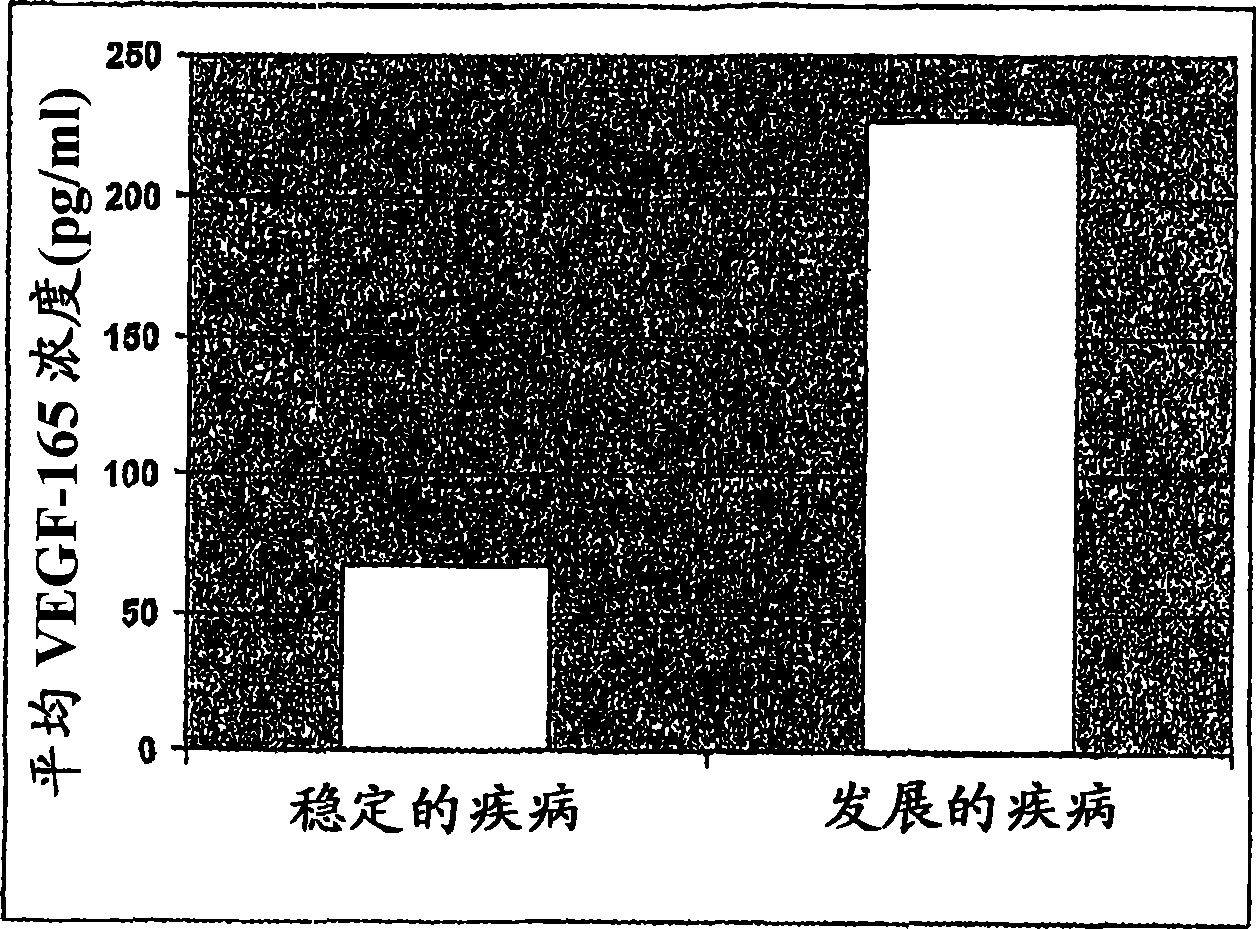
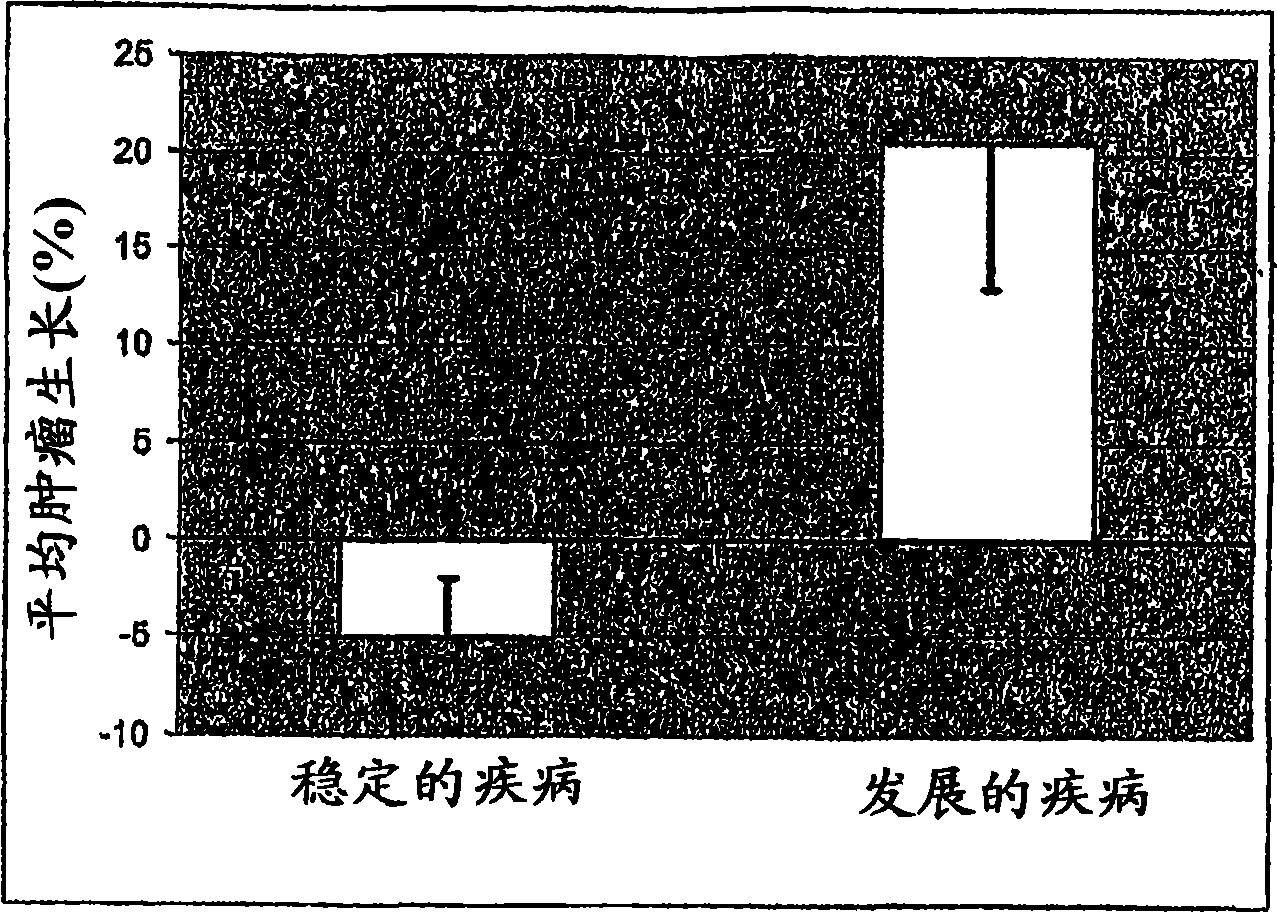
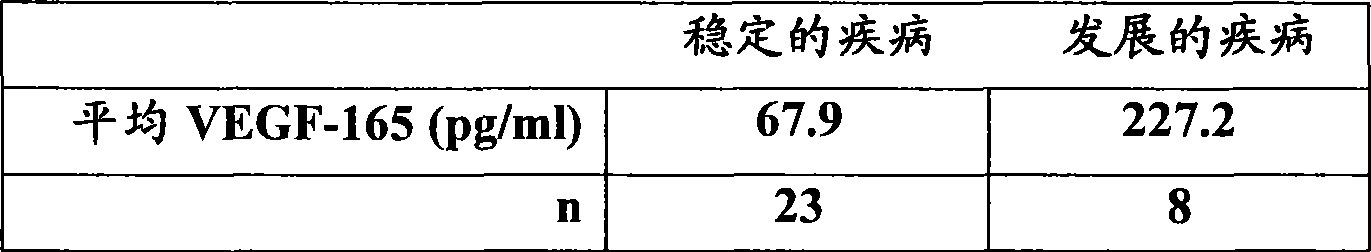
[0111] [087] VEGF-165 levels were determined in duplicate samples using the Oncogene Science VEGF-165 ELISA (Oncogene Science, Cambridge, MA) according to the manufacturer's instructions. The mean of two measurements was determined for each patient. The mean VEGF-165 levels of the 31 patients in this study are reported in Table 1. Table 2 shows the radiometric mean tumor shrinkage in each patient group. The results indicated that the mean VEGF-165 level in patients who showed stable disease following treatment with response to Sorafenib was 67.9 pg / ml. Those patients who still showed progressive disease after Sorafenib treatment had a mean VEGF-165 level of 227.2 pg / ml. Those patients showing stable disease had a mean tumor shrinkage of 5.1%, while those with progressive disease had a mean tumor growth of 20.6%. These results can be shown in figure 1 and 2 middle.
[0112] Table 1: VEGF...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com