Two compounds with quinoxaline mother ring and preparation thereof
A quinoxaline mother and compound technology, applied in the field of new compounds and their preparation, can solve problems such as drug residues, reproductive toxicity, etc., and achieve the effects of improving utilization rate, increasing feed intake, and increasing daily weight gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
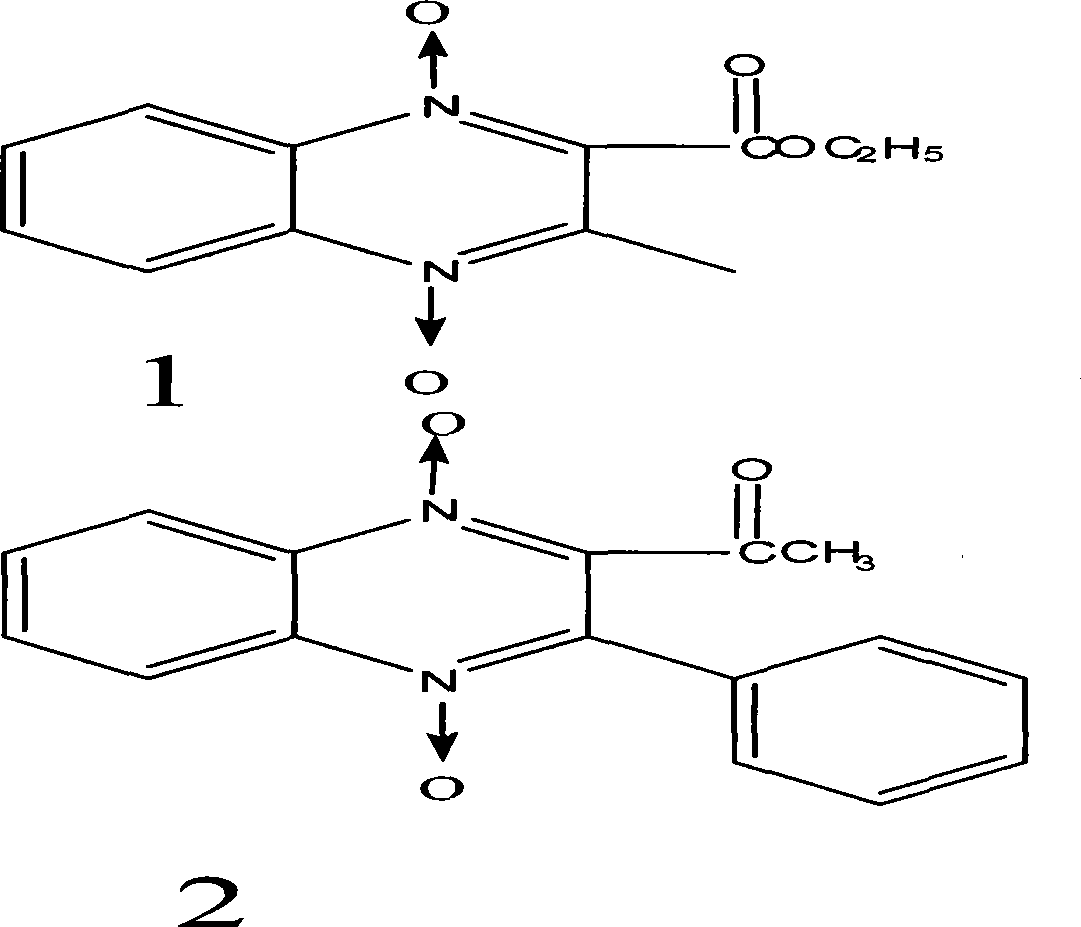
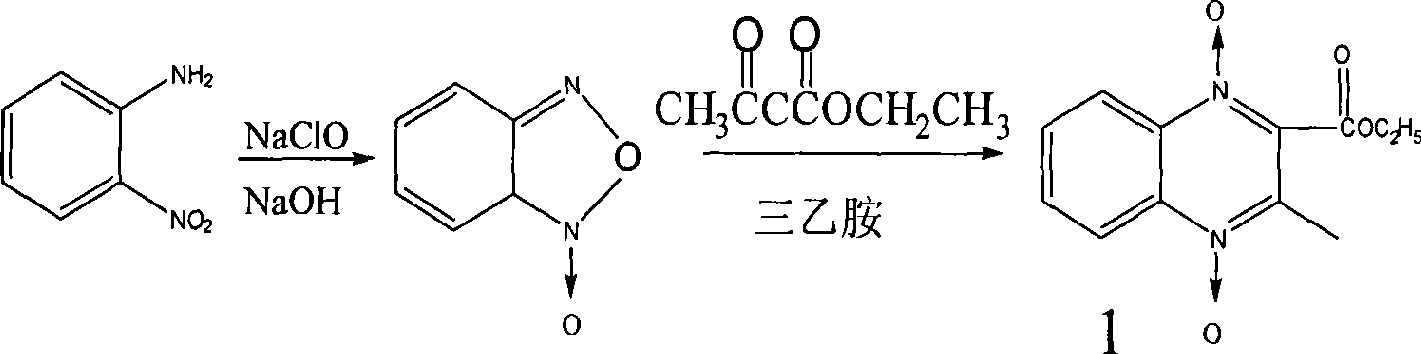
[0076] Synthesis of compound 1
[0077] Add 7g of BFO, add 10g of ethyl acetoacetate, dissolve the BFO in a water bath at 80°C, add 50ml of triethylamine at the same time, shake and place at room temperature for 2-3 days, crystals precipitate, filter with suction, wash twice with a small amount of water, collect the crystals, After drying, the yield is 7g, and the yield is 55%. The reaction mother liquor can continue to precipitate crystals at room temperature, and the combined yield can reach 65%. The crude product can be recrystallized from acetone. Melting point 136-137℃, light yellow needle crystal.
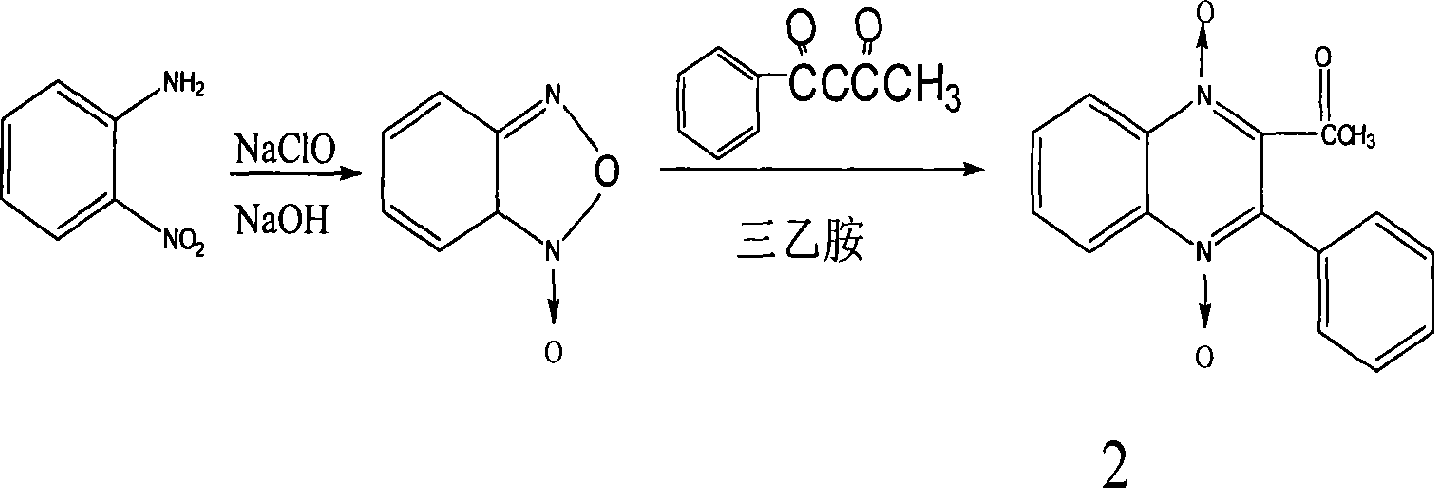
[0078] Synthesis of compound 2
[0079] Dissolve 8g of benzoylacetone in 20ml of absolute ethanol, bathe at 50°C, add 7g of BFO to dissolve, add 50ml of triethylamine at the same time, shake and place at room temperature, a large amount of crystals precipitate after 1 hour, filter with suction, wash with absolute ethanol , dried, yield 10g, yield 71%, melting point 198°C, ...
Embodiment 2
[0081] Synthesis of compound 1
[0082] Add 7g of BFO, add 10g of ethyl acetoacetate, dissolve the BFO in a water bath at 40°C, add 40ml of triethylamine at the same time, shake and place at room temperature for 2-3 days, crystals precipitate, filter with suction, wash twice with a small amount of water, collect the crystals, After drying, the yield is 7g, and the yield is 55%. The reaction mother liquor can continue to precipitate crystals at room temperature, and the combined yield can reach 65%. The crude product can be recrystallized from acetone. Melting point 136-137℃, light yellow needle crystal.
[0083] Synthesis of compound 2
[0084] Dissolve 8g of benzoylacetone in 20ml of absolute ethanol, bathe in water at 40°C, add 7g of BFO to dissolve, add 40ml of triethylamine at the same time, shake and place at room temperature, a large amount of crystals precipitate after 1 hour, filter with suction, wash with absolute ethanol , dried, yield 10g, yield 71%, melting poin...
Embodiment 3
[0086] Synthesis of compound 1
[0087] Add 7g of BFO, add 10g of ethyl acetoacetate, dissolve the BFO in a water bath at 60°C, add 60ml of triethylamine at the same time, shake and place at room temperature for 2-3 days, crystals precipitate, filter with suction, wash twice with a small amount of water, collect the crystals, After drying, the yield is 7g, and the yield is 55%. The reaction mother liquor can continue to precipitate crystals at room temperature, and the combined yield can reach 65%. The crude product can be recrystallized from acetone. Melting point 136-137℃, light yellow needle crystal.
[0088] Synthesis of compound 2
[0089] Dissolve 8g of benzoylacetone in 20ml of absolute ethanol, put in a water bath at 45°C, add 7g of BFO to dissolve, add 60ml of triethylamine at the same time, shake and place at room temperature, a large amount of crystals will precipitate after 1 hour, filter with suction, and wash with absolute ethanol , dried, yield 10g, yield 71%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com