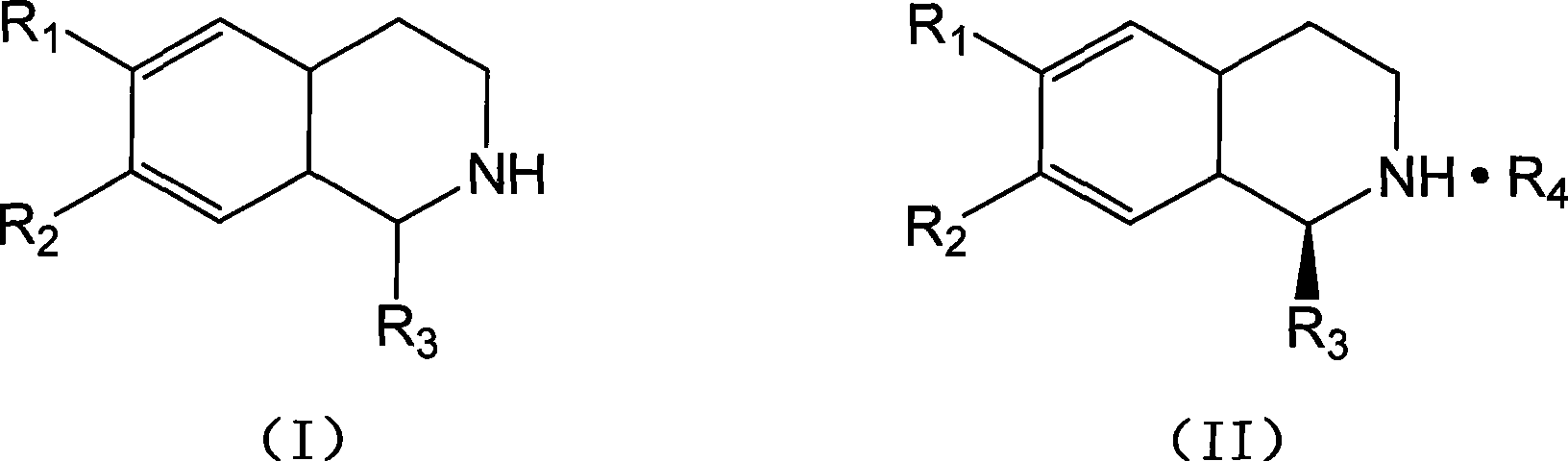
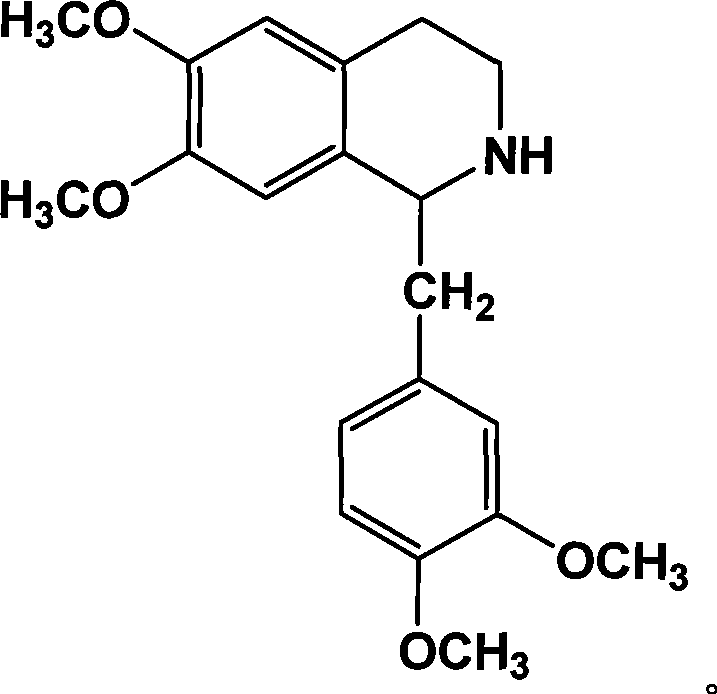
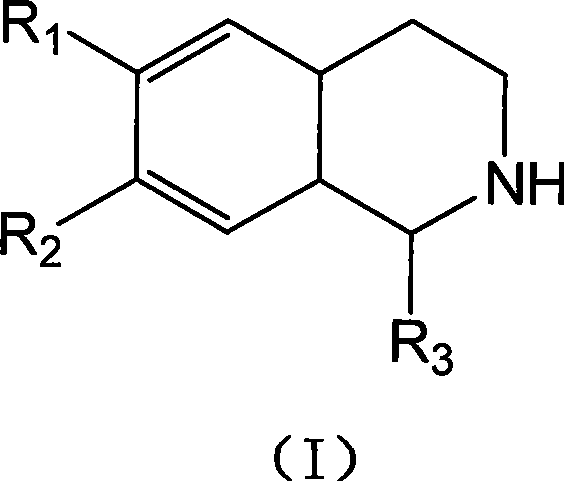
Mixed solvent crystallization resolution method for tetrahydrochysene isoquinoline racemate
A technology of tetrahydroisoquinoline and racemate, which is applied in the direction of organic chemistry, can solve the problems of complex operation, unfavorable industrial scale-up, and long splitting time, so as to simplify the operation process, simplify equipment, and shorten the splitting time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Add 500 g of tetrahydropapaverine oil, 10 L of methanol, and 250 g of N-acetyl-L leucine into a 20 L glass reactor, start stirring, heat to 60 ° C, and react for 40 to 60 minutes. After the end, the temperature was lowered, and the reaction solution was added into a 50L glass reactor with 40L ether, stirred, and a white solid was precipitated, filtered, and dried to obtain 250 g of R-type tetrahydroisoquinoline salt crude product.
[0040] 5L of methanol dissolves the above-mentioned crude product, and after dissolving, it is added into a 50L glass reactor with 20L of ether, stirred, and a white solid is precipitated, filtered, and dried to obtain 110g of R-type tetrahydroisoquinoline salt, and the HPLC detection R% content is greater than 95%, yield 30%.
Embodiment 2
[0041] Embodiment 2: substantially identical with embodiment 1, but wherein the polar solvent described in (1) step uses acetone instead; Polar solvent: tetrahydroisoquinoline racemate=1:60. The non-polar solvent is changed to petroleum ether; polar solvent / non-polar solvent=1:25. The ratio of tetrahydroisoquinoline racemate to resolving agent adopts a molar ratio of 1:1.
Embodiment 3
[0042] Embodiment 3: substantially the same as Example 1, but wherein the polar solvent described in (1) step uses ethanol instead; Polar solvent: Tetrahydroisoquinoline racemate=1:10; Resolving agent Use N-acetyl-D-leucine instead; use isopropyl ether as the non-polar solvent; polar solvent / non-polar solvent=1:10. The ratio of the tetrahydroisoquinoline racemate to the resolving agent is a molar ratio of 1:1.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com