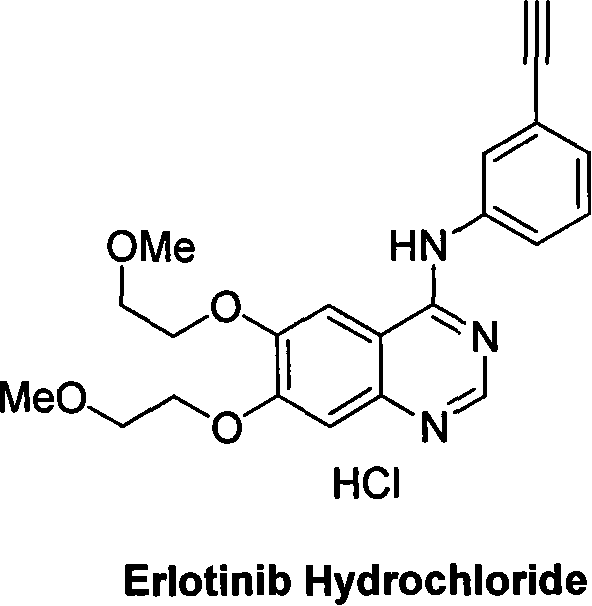
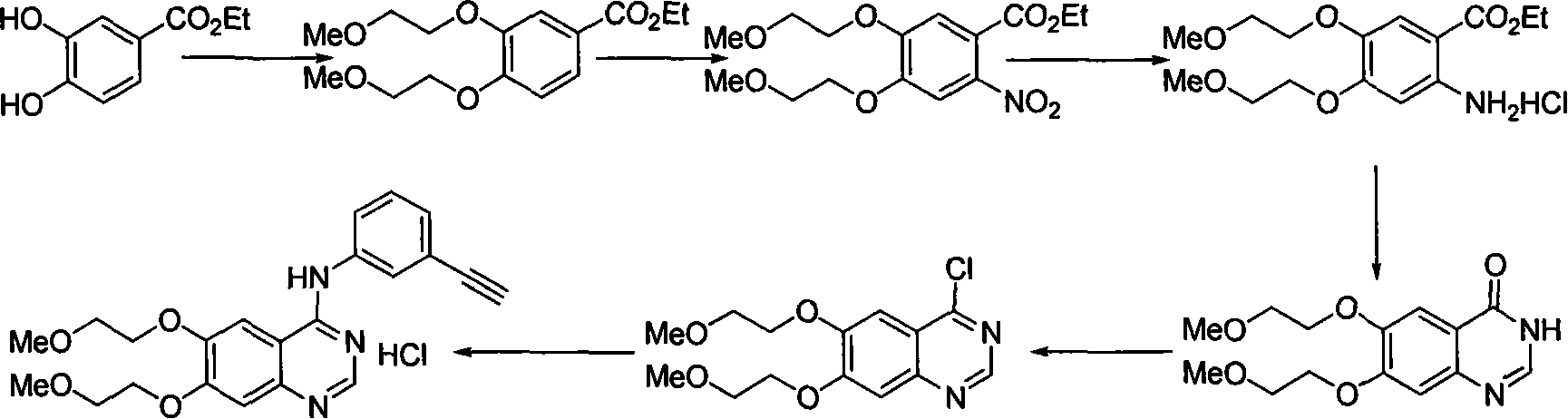
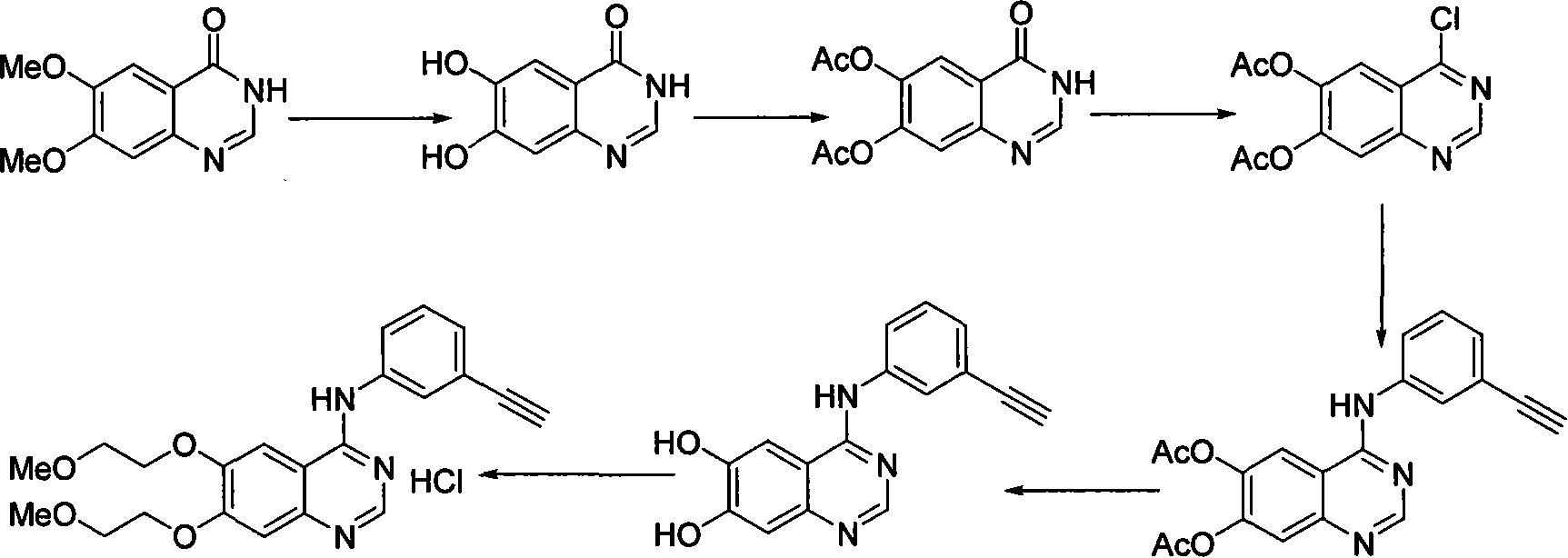
Preparation of erlotinid hydrochloride
A technology of erlotinib hydrochloride and mixed acid, applied in the field of hydrochloric acid 4--6, can solve the problems such as reducing yield and increasing steps, and achieves the effects of avoiding excessive hydrolysis, mild reaction conditions and shortening synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] ①Synthesis of Compound 1
[0041] Add 5L of double-distilled toluene to a 10L four-necked bottle, add 1kg of raw materials 3,4-dihydroxybenzaldehyde, 500g of hydroxylamine hydrochloride, 160g of p-toluenesulfonic acid and 5kg of anhydrous magnesium sulfate under stirring, heat and reflux for 6 hours, cool to room temperature, Filtration, the filter cake was extracted with hot ethyl acetate to TLC to detect no product, combined, washed with water, washed with saturated brine, dried, concentrated until a large amount of product was precipitated, added 2.5L petroleum ether, suction filtered, washed with a small amount of petroleum ether, and dried to obtain The compound is about 1800g.
[0042] ② Synthesis of compound 2
[0043] Add compound 1 (1kg), chloroethyl methyl ether 2kg, potassium carbonate 3kg, tetrabutylammonium iodide 500g and 5 liters of DMSO into a 10-liter four-neck flask, heat to reflux, and after TLC detects that the reaction is complete, cool and pour 10...
Embodiment 2
[0055] ①Synthesis of Compound 1
[0056] Add 5L double-distilled benzene into a 10L four-necked bottle, add 1kg of raw materials 3,4-dihydroxybenzaldehyde, 1kg of hydroxylamine hydrochloride, 100g of p-toluenesulfonic acid and 4kg of anhydrous magnesium sulfate under stirring, heat and reflux for 16 hours, cool to room temperature, Filtration, the filter cake was extracted with hot ethyl acetate to TLC to detect no product, combined, washed with water, washed with saturated brine, dried, concentrated until a large amount of product was precipitated, added 2.5L petroleum ether, suction filtered, washed with a small amount of petroleum ether, and dried to obtain The compound is about 1850g.
[0057] ② Synthesis of compound 2
[0058]Add compound 1 (1kg), chloroethyl methyl ether 2kg, potassium carbonate 3kg, tetrabutylammonium bromide 100g and 5 liters of DMF into a 10-liter four-neck flask, heat to reflux, and after TLC detects that the reaction is complete, cool and pour 10 ...
Embodiment 3
[0070] ①Synthesis of Compound 1
[0071] Add 5L double-distilled toluene to a 10L four-necked bottle, add 1kg of raw materials 3,4-dihydroxybenzaldehyde, 500g of hydroxylamine hydrochloride, 160g of p-toluenesulfonic acid and 5kg of anhydrous sodium sulfate under stirring, heat and reflux for 6 hours, cool to room temperature, Filtration, the filter cake was extracted with hot ethyl acetate to TLC to detect no product, combined, washed with water, washed with saturated brine, dried, concentrated until a large amount of product was precipitated, added 2.5L petroleum ether, suction filtered, washed with a small amount of petroleum ether, and dried to obtain The compound is about 1900g.
[0072] ② Synthesis of Compound 2
[0073] Add compound 1 (1kg), chloroethyl methyl ether 2kg, potassium carbonate 5kg, tetrabutylammonium iodide 300g and 5 liters of THF into a 10 liter four-neck flask, heat to reflux, and after TLC detects that the reaction is complete, cool and pour 10 liter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com