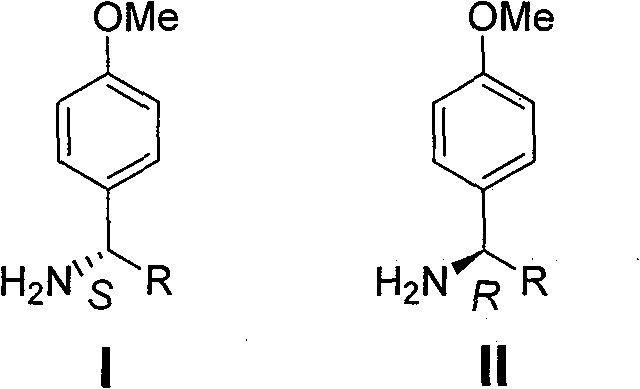
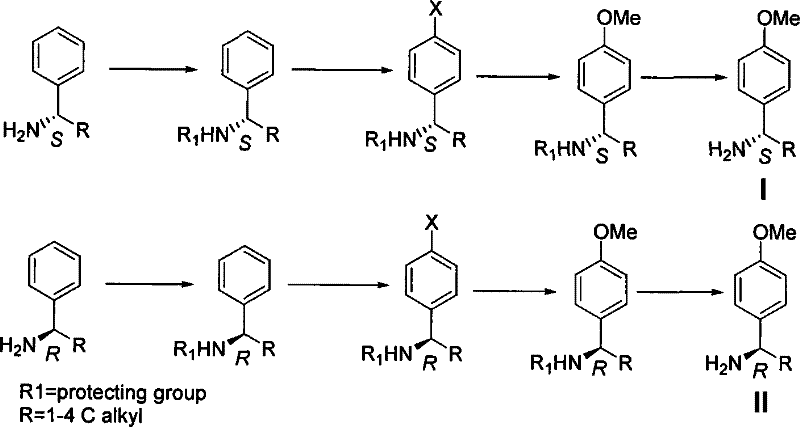
The synthetic method of chiral p-methoxybenzylamine
The technology of a kind of methoxybenzylamine and the synthesis method is applied in the field of synthesis of chiral p-methoxybenzylamine, and achieves the effects of being beneficial to industrial production, easy to crystallize and purify, and easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In a 10-liter four-neck flask, add 5 liters of anhydrous methanol and 1.21 kg of S-phenylethylamine, and after stirring at room temperature for 15 minutes, add 1.1 kg of acetic anhydride dropwise. After the addition is completed, stir at room temperature, concentrate after the TLC detection reaction, and obtain The solid was washed with water, and vacuum-dried to obtain 1.6kg of product, which was directly used in the next reaction.
[0028] Dissolve 1.6 kg of the product in the previous step in 5 liters of dichloromethane, add 1.5 kg of dibromohydantoin in batches, and react overnight at room temperature after adding, pour into 5 liters of ice water, extract the reaction with 10% sodium sulfite solution, and separate the liquids , conventional post-processing to obtain 1.1 kg of white solid product, which was directly used in the next reaction.
[0029] Dissolve 1.1 kg of the product in the previous step in 2 liters of methanol and 2 liters of DMF, add 2 kg of sodium m...
Embodiment 2
[0032] Add 5 liters of anhydrous methanol and 1.21 kg of S-phenylethylamine to a 10-liter four-necked flask, stir at room temperature for 15 minutes, then add 2.5 kg of trifluoroacetic anhydride dropwise, complete the addition, stir at room temperature, and concentrate after TLC detection , the obtained solid was washed with water, and vacuum-dried to obtain 2.1 kg of product, which was directly used in the next reaction.
[0033] Dissolve 2.1 kg of the product from the previous step in 5 liters of dichloromethane, add 1.5 kg of dibromohydantoin in batches, and react overnight at room temperature after adding, pour into 5 liters of ice water, extract the reaction with 10% sodium sulfite solution, and separate the liquids , conventional post-processing to obtain 1.5kg of white solid product, which was directly used in the next reaction.
[0034] Dissolve 1.5 kg of the product in the previous step in 2 liters of methanol and 2 liters of DMF, add 2 kg of sodium methoxide, 300 g o...
Embodiment 3
[0037] In a 10-liter four-neck flask, add 5 liters of anhydrous methanol and 1.21 kg of S-phenylethylamine, and after stirring at room temperature for 15 minutes, add 1.1 kg of acetic anhydride dropwise, complete the addition, stir at room temperature, concentrate after the TLC detection reaction, and obtain The solid was washed with water, and vacuum-dried to obtain 1.6kg of product, which was directly used in the next reaction.
[0038] Dissolve 1.6 kg of the product in the previous step in 5 liters of dichloromethane, add 1.3 kg of iodine in batches, and react overnight at room temperature after adding, pour into 5 liters of ice water, extract the reaction with 10% sodium sulfite solution, separate the liquids, and The white solid product 1.5kg obtained was directly used in the next reaction.
[0039] Dissolve 1.5 kg of the product in the previous step in 2 liters of methanol and 2 liters of DMF, add 2 kg of sodium methoxide, 300 g of cuprous cyanide, heat and reflux for 6 ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap