Synthetic method of bromamines muscle relaxant
A muscle relaxant and a synthesis method technology, applied in the production of steroids, bulk chemicals, organic chemistry, etc., can solve problems such as affecting product purity, improve product yield and product quality, reduce costs, and simplify operations. effect of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
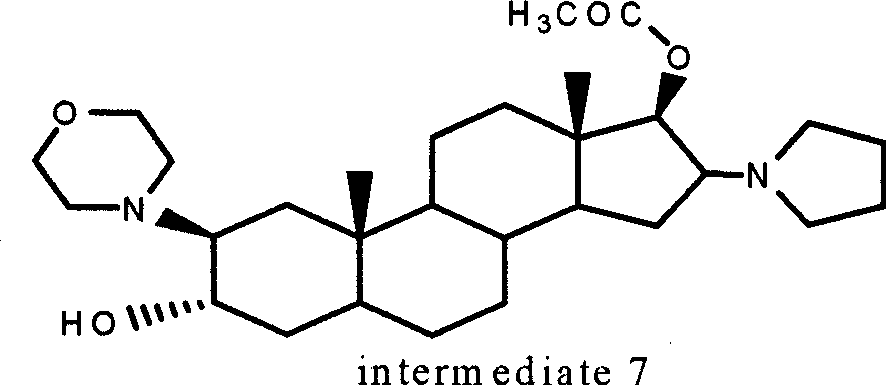
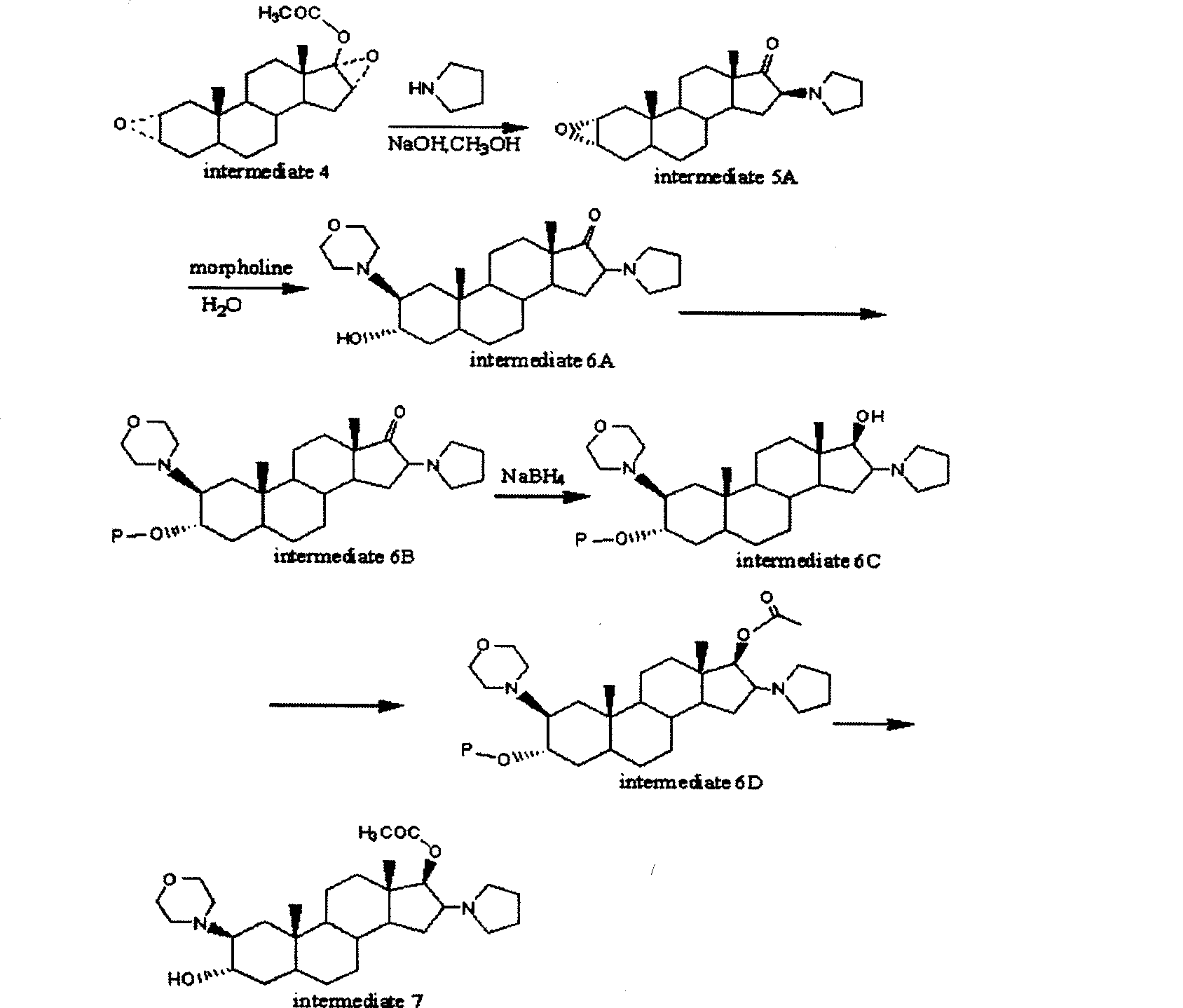
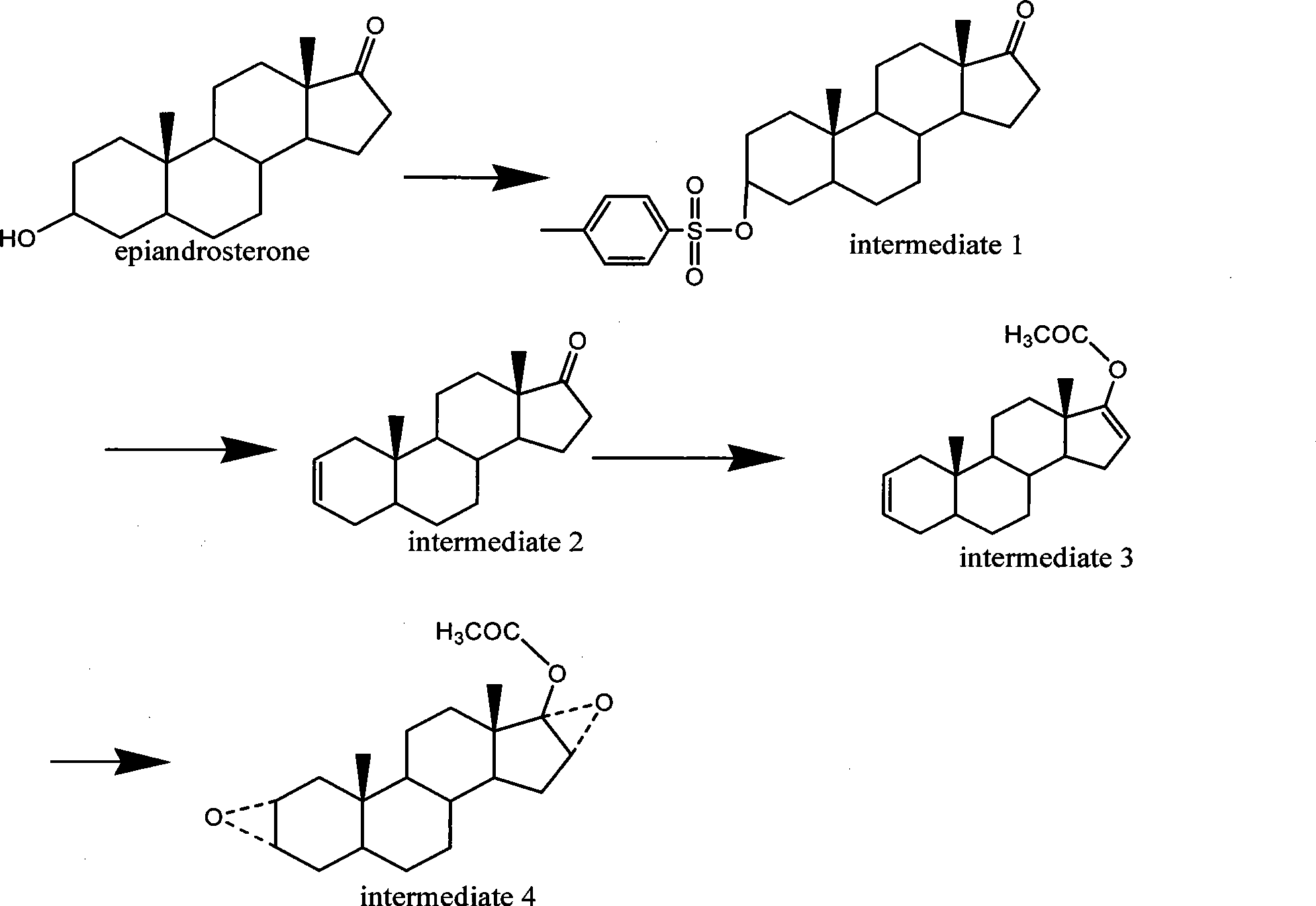
[0050] Example 12α, 3α, 16α, the synthesis of 17α-dioxyoxy-17-acetoxy-5-androstane (intermediate 4)
[0051] 1.1.5 Preparation of α-androst-2-en-17-one (interemediate 2)
[0052] Add 100g of epiandrosterone, 30ml of pyridine, 600ml of dichloromethane, 120g of p-toluenesulfonyl chloride into the reaction bottle, heat and reflux for 3h; Drying over sodium sulfate and concentrating under reduced pressure gave 144 g of the white body 2-p-phenylmethylsulfonyl-epiandrosterone (intermediate 1) with a yield of 92%;
[0053] Mix 60 g of the above-mentioned yellow-white solid and 80 ml of DMSO and heat to 120° C., and stir for 3 h. Stop heating, stand at room temperature for crystallization overnight, filter, wash the filter cake with water, and dry to obtain 31 g of a flesh-colored solid of 5α-androst-2-en-17one with a melting point of 102-105°C and a reaction yield of 87%.
[0054] 1.2.1 Synthesis of 7-acetoxy-5α-androst-2,16-diene (intermediate 3)
[0055] Add 50g of 5α-androst-2-...
Embodiment 2
[0058] The preparation of embodiment 2 rocuronium bromide
[0059] 2.1.2 Preparation of β-(4-morpholinyl)-16β-(1-pyrrolidinyl)-5α-androst-3α-ol, 17β-acetate
[0060] Dissolve 10 g of 2α, 3α, 16α, 17α-diepoxy-17β-acetoxy-5-androstane (intermediate 4) prepared by the method in Example 1 above in 100 ml of methanol, add 10 ml of 4N sodium hydroxide , heated to reflux for 30 minutes, the solution was cooled to 40°C, 15 ml of tetrahydropyrrole was added and heated to reflux for 15 minutes, cooled, filtered, washed with water until neutral, and a white solid was obtained. Put the solid into a stainless steel pressure reactor, add 50ml of morpholine and 5ml of water into the reactor, and react at 140°C for 36 hours; after cooling, the solvent is evaporated under reduced pressure, and the residue is crystallized with acetone to obtain 4.5g of white crystals. This compound was identified as Intermediate 6A.
[0061] Add 4.5 g of the above-mentioned intermediate 6A into a 50 ml reacti...
Embodiment 3
[0073] The preparation of embodiment 3 vecuronium bromide
[0074] Preparation of 2β, 16β-dipiperidinyl-5α-androst-3α-ol-17-one (intermediate 8)
[0075] In a pressure reaction vessel lined with polytetrafluoroethylene, add 2α, 3α, 16α, 17α-diepoxy-17β-acetoxy-5-androstane 7g, 70ml of piperidine, and 7ml of water. Place in an oven at 150°C for 40 hours. Cool the reactor to room temperature, open the reactor, take out the reactant, evaporate the solvent under reduced pressure, dissolve the residue with 2N HCl and filter, adjust the filtrate to pH 8-10 with 10% NaOH, filter out the solid, wash it with water, dry it with acetone Crystallized to obtain 6 g of white crystals.
[0076] By 2β, 16β-dipiperidinyl-5α-androst-3α-ol-17-ketone (intermediate 8) can refer to the specific method of preparing vecuronium bromide (Zhu Baoquan. New Drug Synthesis Handbook. Beijing: Chemical Industry Press 2002.12).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com