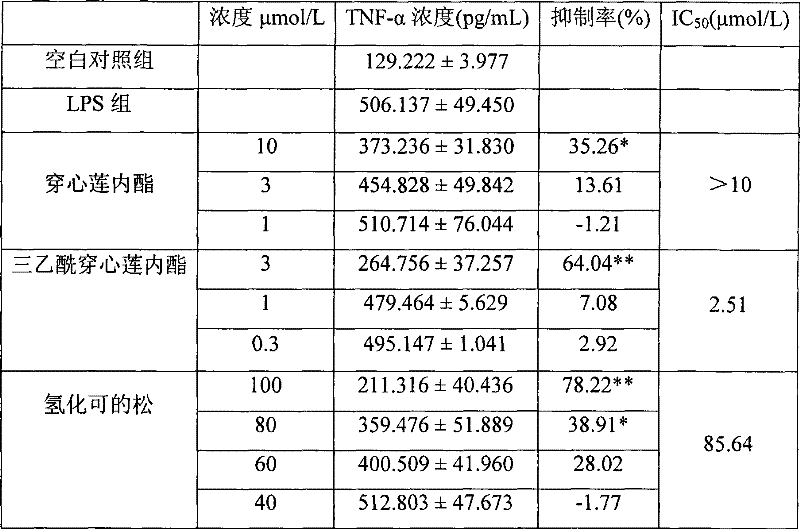
Medical use of triacetyl andrographolide as proinflammatory cytokine inhibitor
A technology of inflammatory cytokines and andrographolide, which is applied in the field of medical application of triacetylandrographolide as an inhibitor of inflammatory cytokines, can solve the problem that the inhibitory effect and activity of triacetylandrographolide cannot be speculated on inflammatory cytokines How to wait for the problem to achieve the effect of improving the inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0028] The preparation of preparation example 1 andrographolide
[0029] Take 20 g of the crude product of andrographolide, dissolve it in 450 mL of methanol at 72° C., let it stand for 24 hours to obtain white crystals, and then dry them to obtain andrographolide.
preparation example 2 3
[0030] The preparation of preparation example 2 triacetylandrographolide
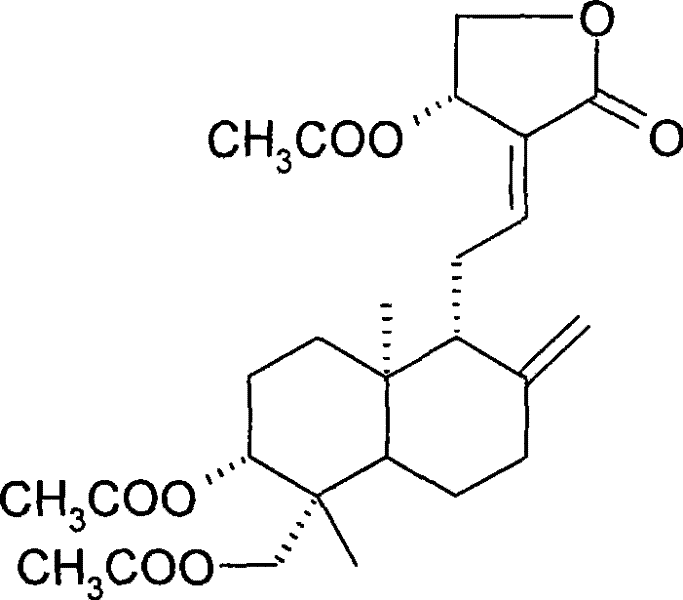
[0031] 1g andrographolide dissolved in 10mLAc 2 O, heated to reflux for 2 hours, the reaction mixture was extracted with ethyl acetate, the organic layer was successively water, saturated NaHCO 3 and saturated brine, anhydrous MgSO 4 dry. Triacetylandrographolide was obtained as a white solid by silica gel column chromatography. The molecular formula is: C 26 h 36 o 8 .
[0032] Compound confirmation data are as follows:
[0033] 3454, 3080, 2954, 1731, 1683, 1375, 1250, 1191, 1075, 1026, 895, 606.
[0034] 1 H (400MHz, CDCl 3 ):δ
[0035] 7.00 (td, J=6.84, 1.48Hz, 1H, H-12), 5.92 (d, J=5.96Hz, 1H, H-14), 4.90 (bs, 1H, H-17a), 4.60 (dd, J =11.68, 4.26Hz, 1H, H-15a), 4.55(dd, J=11.24, 6.08Hz, 1H, H-15b), 4.53(bs, 1H, H-17b), 4.36(d, J=11.80Hz , 1H, H-19a), 4.25(dd, J=11.24, 1.80Hz, 1H, H-3), 4.12(d, J=11.80Hz, 1H, H-19b), 2.52~2.37(m, 3H) , 2.12(s, 3H), 2.05(s, 6H), 2.00~1.96(m, 1H), 1.90...
preparation example 33,19- 2
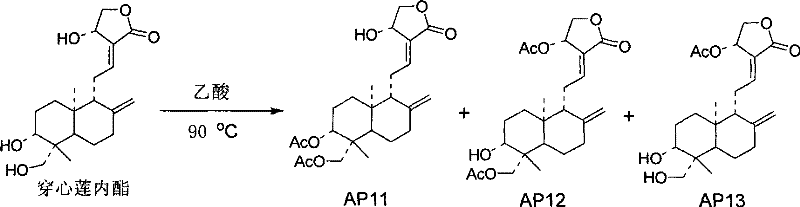
[0039] Preparation Example 33, Preparation of 19-diacetylandrographolide, 14-acetylandrographolide, and 14,19-diacetylandrographolide
[0040] 5g of andrographolide was added to 30mL of acetic acid, stirred and heated to 80°C, and reacted overnight. Extracted with ethyl acetate, the organic layer was washed with water and saturated brine successively, anhydrous MgSO 4 dry. 3,19-diacetylandrographolide (AP11), 14,19-diacetylandrographolide (AP12), and 14-acetylandrographolide (AP13) were obtained by column chromatography.
[0041]
[0042] The confirmed data of AP11 compound are as follows:
[0043] Molecular formula: C 24 h 34 o 7 (434)
[0044] 1 H (400MHz, CDCl 3 ): 6.97 (1H, m, H-12), 5.03 (1H, d, J=5.6Hz, H-14), 4.92 (1H, s, H-17), 4.62 (1H, s, H-17) , 4.59 (1H, dd, J=11.2, 4.8Hz, H-3), 4.47 (1H, dd, J=10.4, 6.0Hz, H-15), 4.38 (1H, d, J=11.6Hz, H- 19), 4.26 (1H, dd, J=10.4, 2.0Hz, H-15), 4.11 (1H, d, J=12.0Hz, H-19), 2.05 (6H, s, CH3CO-), 1.04 (3H , s, H-18),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com