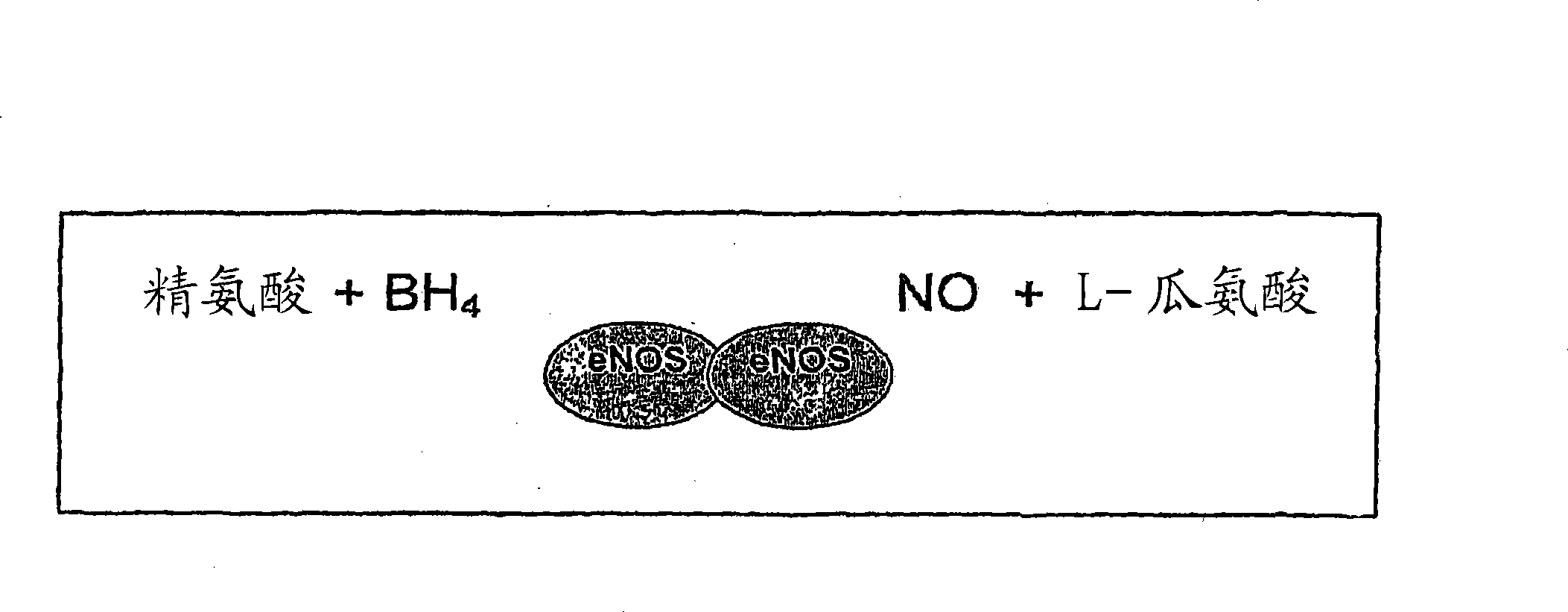
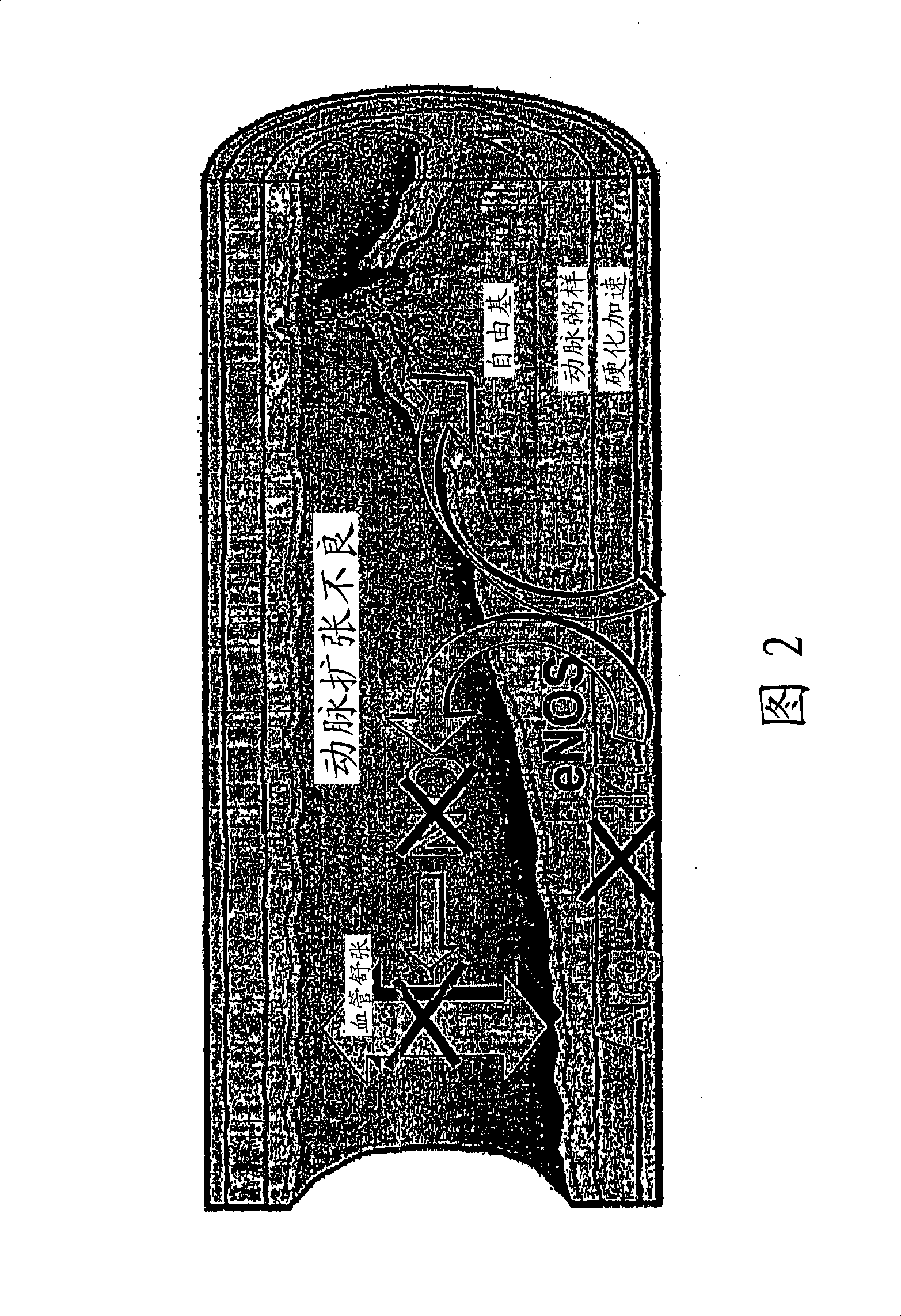
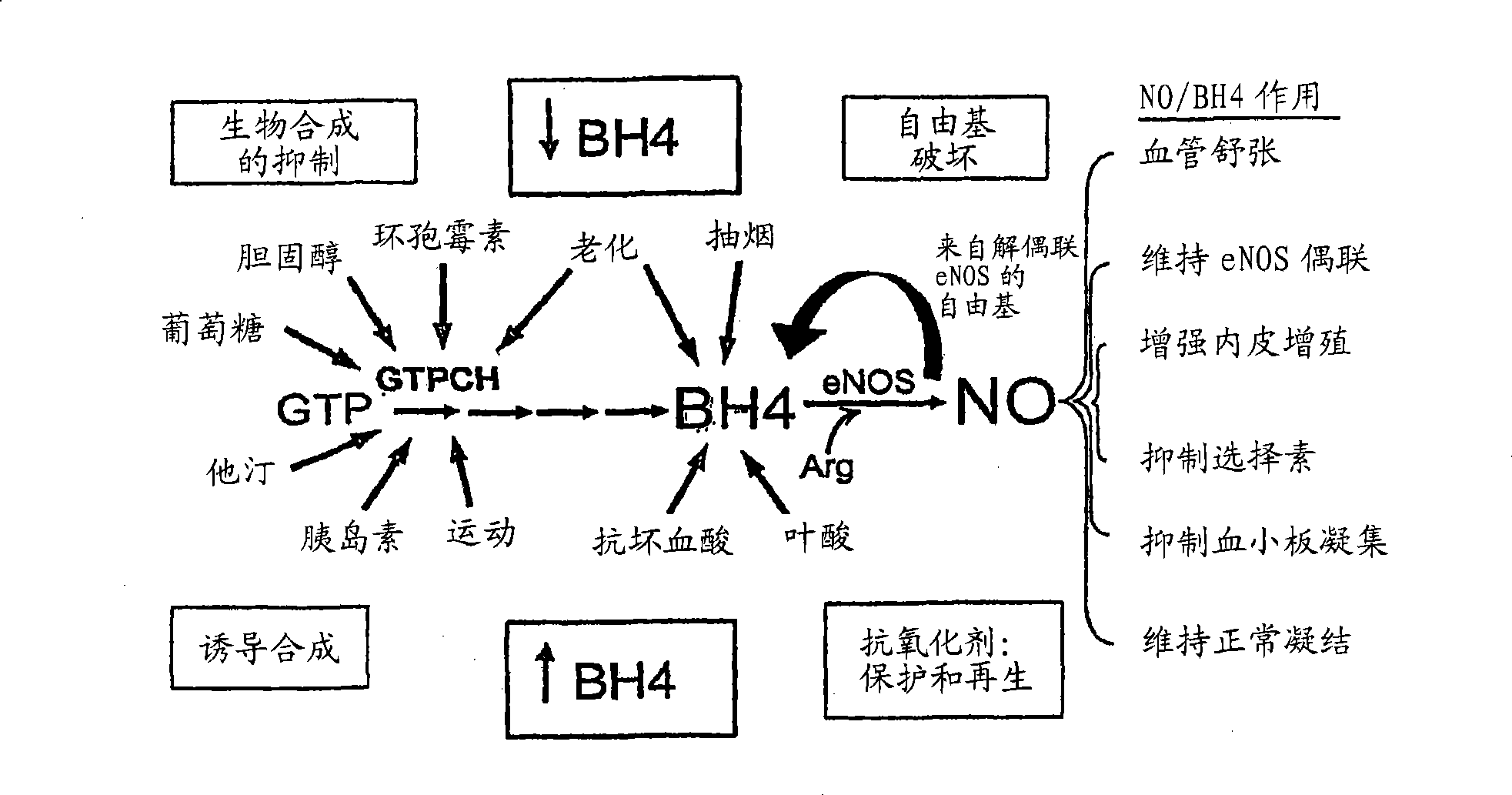
Methods and compositions for the treatment of vascular disease
A technology for the treatment of drugs and solvates, which is applied in drug combinations, cardiovascular system diseases, and pharmaceutical formulations, and can solve problems such as non-specificity, lack of patient compliance with treatment plans, and limited curative effect range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0223] For the preparation of polymorphs, crystallization techniques well known in the art may be employed, such as stirred suspensions (phase equilibration), precipitation, recrystallization, evaporation, adsorption of solvents (eg water) or decomposition of solvates. Crystallization can be carried out from a dilute, saturated or supersaturated solution, with or without a suitable nucleating agent as a seed. Temperatures up to 100°C may be employed to form the solution. Cooling down to -100°C, preferably down to -30°C may be performed to induce crystallization and precipitation. Metastable polymorphs or pseudopolymorphs can be used to prepare solutions or suspensions that are used to prepare more stable forms, and to achieve higher solution concentrations.
[0224] Surprisingly, hydrate form D was found to be the most stable form of hydrates, with forms B and D being particularly suitable for use in pharmaceutical formulations. Forms B and D possess advantages such as targe...
Embodiment 1
[0274] Clinical evaluation of 6R-tetrahydrobiopterin
[0275] The following examples provide guidance for the parameters used in the clinical evaluation of BH4 in the treatment methods of the present invention. As described elsewhere herein, BH4 will be used to treat diabetes-related and non-diabetic cardiovascular complications including, but not limited to, resistant hypertension, intermittent claudication, coronary hypertension, coronary artery disease, pulmonary Hypertension and hemolytic anemia (including sickle cell disease). A clinical trial will be conducted to provide an evaluation of safe daily oral doses of BH4, pharmacokinetics, initial response to the alternative, and defined clinical endpoints. A minimum of one-week trial per patient (but not necessarily limited to one week) to assess efficacy in reversing relevant study endpoints (e.g., development of pain during ambulation in patients with intermittent claudication) and sufficient data collected from 30 evalua...
Embodiment 2
[0290] Clinical evaluation of 6R-tetrahydrobiopterin in diabetic patients
[0291] Determination of Dose Effect, Dose Interval and Safety in Phase 1 / 2 or 2a Diabetic Patients
[0292] Prior to initiating any phase 2a dose / efficacy study, a shorter phase 1 / 2 dose escalation study in various diabetic populations to determine the dose effect on vascular compliance and safety should be considered. The first study will define the dose range and regimen and the range of vascular function endpoints that can be monitored to support clinical endpoints. Subjects in the first study will be diabetic patients with significant vascular disease (decreased compliance of microvessels) and hypertension, administered BH4 once daily or twice daily, orally Doses were 0 mg / kg, 1 mg / kg, 2 mg / kg, 5 mg / kg, 10 mg / kg and 20 mg / kg. Patients will be monitored for vascular compliance, perfusion / reperfusion / forebrach blood flow and blood pressure during the one-week treatment period and over the span of a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com