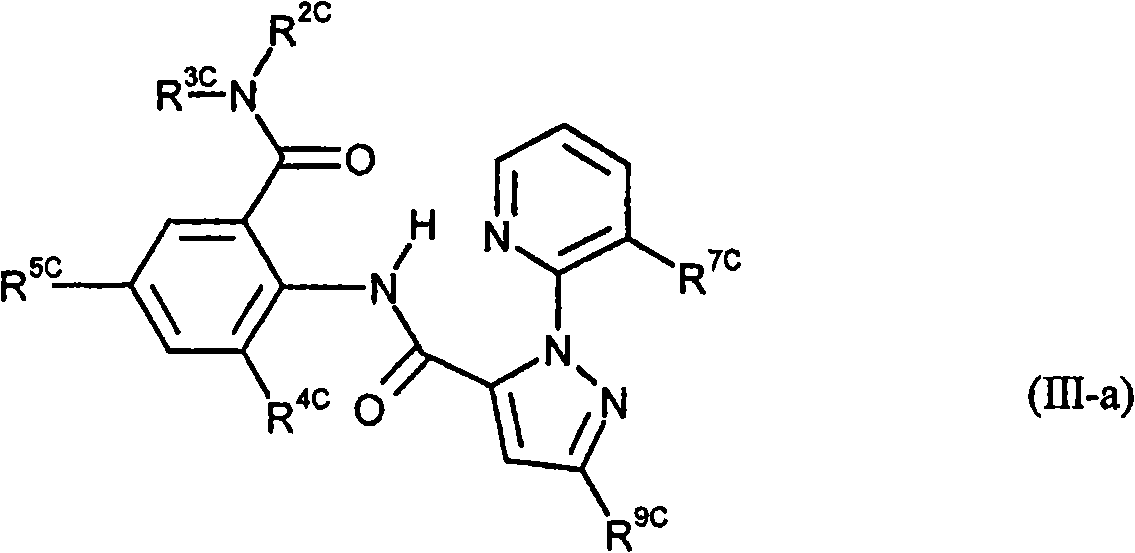
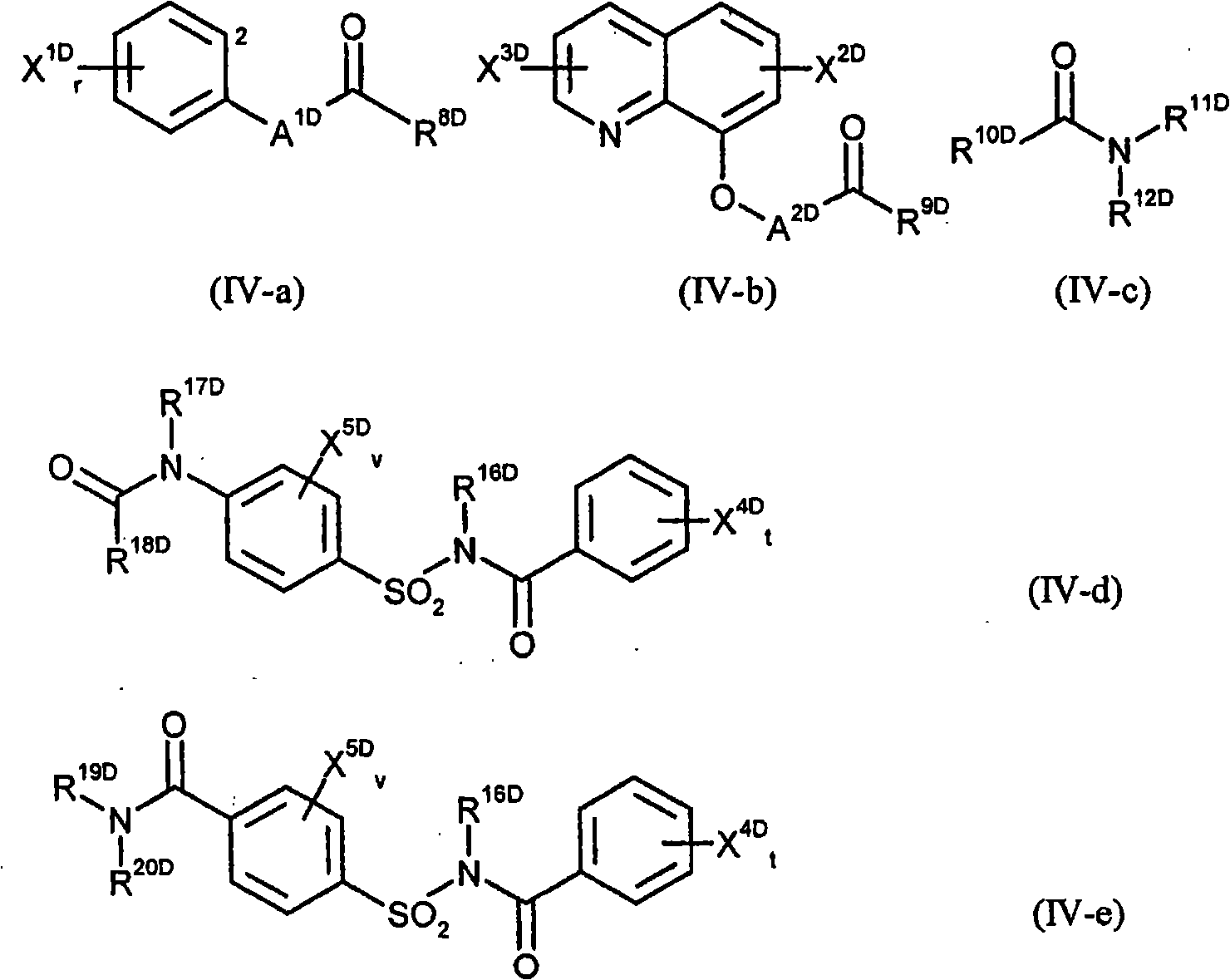
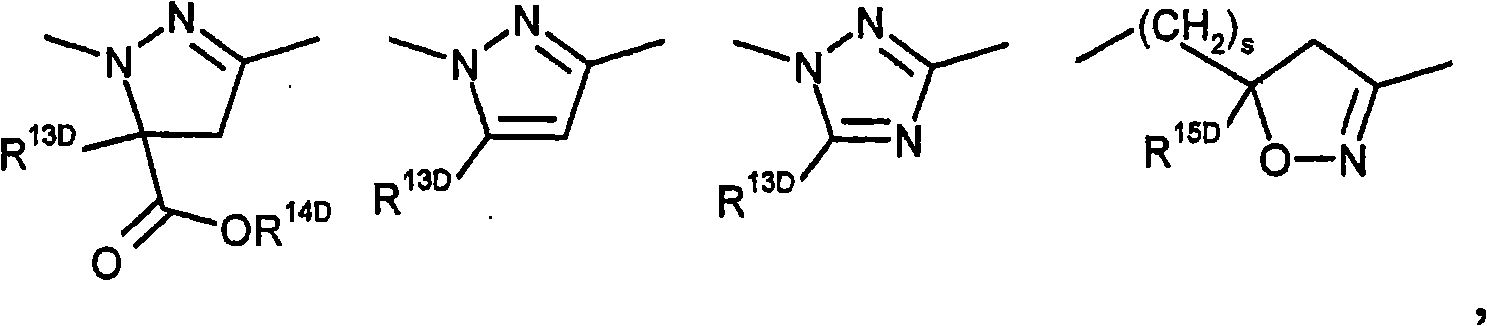
Selective insecticides based on anthranilic acid diamides and safeners
An active, alkyl-based technology for use in biological control chemicals, biocides, biocides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0182] In the definitions above and below, a saturated or unsaturated hydrocarbon group such as an alkyl, alkenyl or alkanediyl group - including in combination with a heteroatom as in an alkoxy group - may each be straight-chain or branched.
[0183] Unless stated otherwise, optionally substituted radicals can be monosubstituted or polysubstituted, where in the case of multiple substitution the substituents can be identical or different.
[0184] C 1 -C 20 The definition of alkyl includes the widest range of alkyl groups defined herein. Specifically, this definition includes methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl, 1-methylbutyl, 2-methyl butylbutyl, 3-methylbutyl, 1-ethylpropyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl, 2,2-dimethylpropyl, and each All isomeric hexyl groups (such as n-hexyl, 1-methylpentyl, 2-methylpentyl, 3-methylpentyl, 4-methylpentyl, 1,2-dimethylbutyl , 1,3-dimethylbutyl, 2,3-dimethylbutyl, 1,1-dimethylbut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com