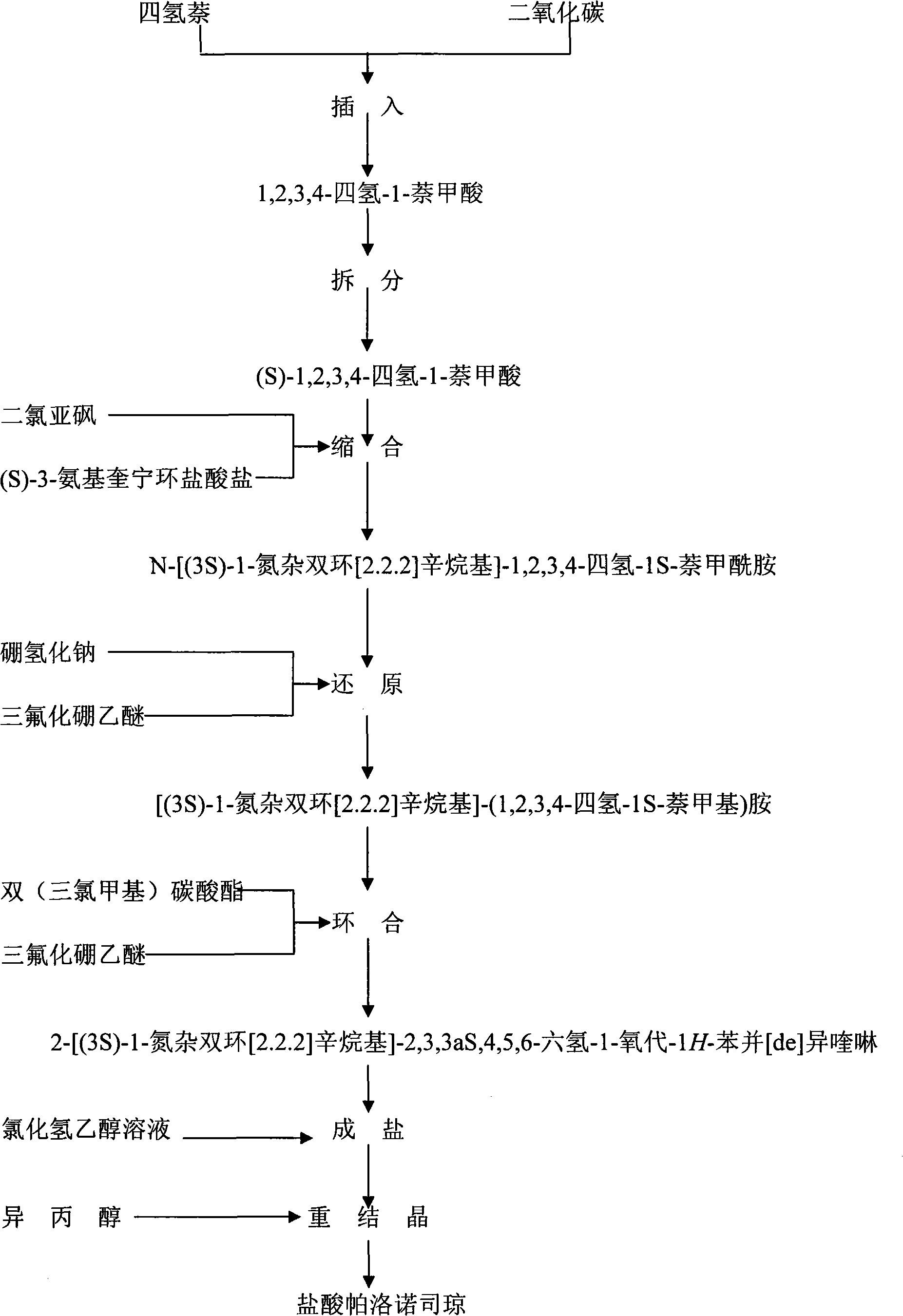
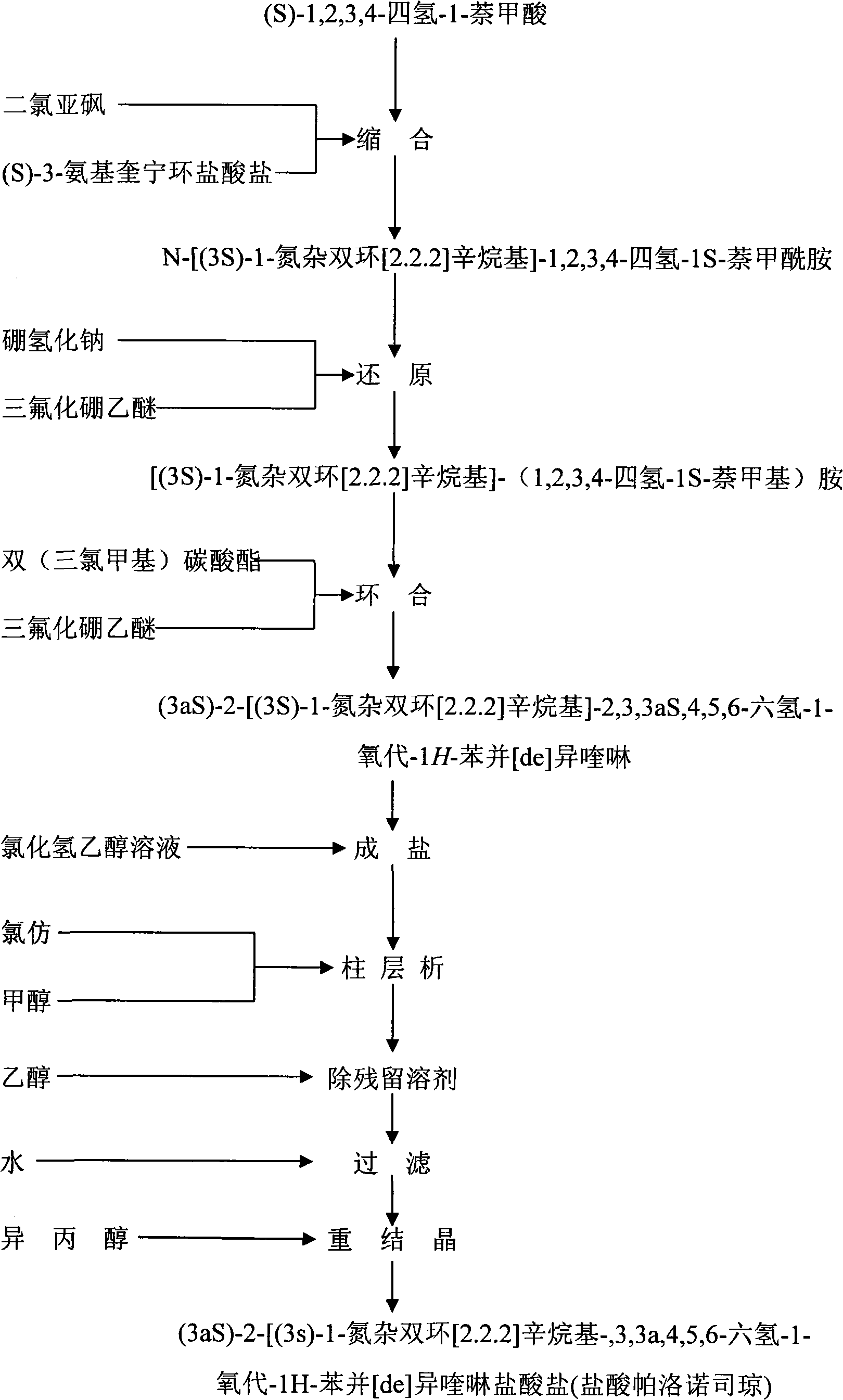
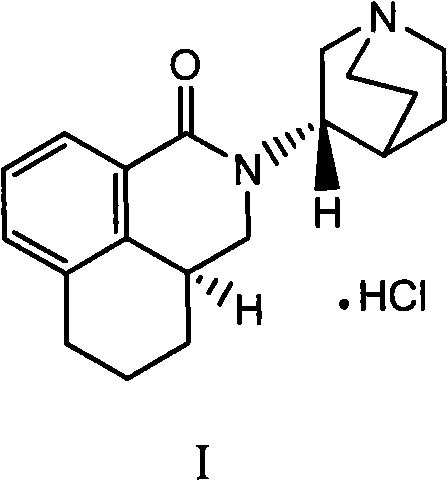
Preparation process of high-purity palonosetron Hcl
A technology of palonosetron and preparation process, which is applied in the field of organic synthesis, can solve the problems of difficult product quality, multiple impurities and the like, achieves the effects of quality controllability and product quality improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0040] Example 2 New Method 2 Preparation of Palonosetron Hydrochloride
[0041]Weigh 20.0 g of the pre-column crude product of palonosetron hydrochloride prepared in Example 1, and perform elution according to the elution conditions of Example 1 to prepare 10.0 g of crude product of palonosetron hydrochloride. Dissolve the crude palonosetron hydrochloride in 250mL of chloroform, then add 0.1g of activated carbon, stir at room temperature for 30 minutes, filter and concentrate to dryness, then add 200mL of 95% ethanol, heat to dissolve completely, and then concentrate to dryness under reduced pressure; add 60mL Dissolve in water, pass through a 0.22μm microporous membrane, filter out a small amount of insoluble matter, concentrate the mother liquor under reduced pressure; add 230mL of isopropanol and 9mL of water, heat to dissolve all the solids, then continue to heat, and distill at normal pressure until 70mL of liquid is evaporated Afterwards, the distillation was stopped, c...
Embodiment 3
[0042] Example 3 New Method 3 Preparation of Palonosetron Hydrochloride
[0043] Weigh 20.0 g of the pre-column crude product of palonosetron hydrochloride prepared in Example 1, and perform elution according to the elution conditions of Example 1 to prepare 10.3 g of crude product of palonosetron hydrochloride. Add 250mL of 80% ethanol, heat to dissolve completely, then concentrate to dryness under reduced pressure; then add 100mL of water to dissolve, pass through a 0.8μm microporous membrane, filter out a small amount of insoluble matter, concentrate the mother liquor under reduced pressure, and finally add 45mL×2 anhydrous Add 200 mL of absolute ethanol, heat to dissolve all the solids, cool to room temperature, crystallize in an ice bath for 2 hours, filter, and dry the precipitate under reduced pressure to obtain 9.2 g of the finished product. The purity of the product is 99.82% through HPLC detection, and the single impurity is below 0.05%; through gas phase detection, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com