Long-acting low molecule heparin intraocular sustained-release system
A low-molecular-weight heparin, long-acting technology, applied in medical preparations with non-active ingredients, high-molecular compound non-active ingredients, medical preparations containing active ingredients, etc., to achieve long half-life, overcome bleeding, and high bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
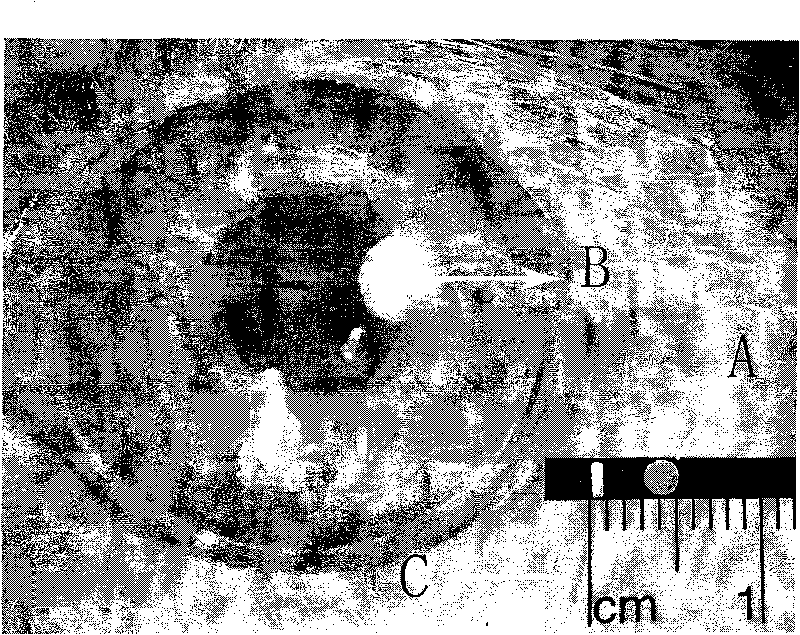
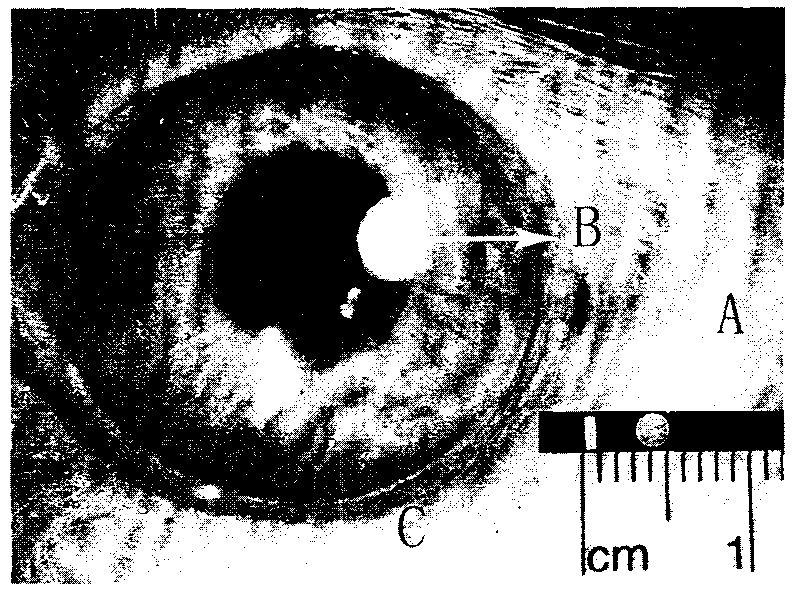
[0017] 10 parts of lactic acid-glycolic acid copolymer (PLGA) (molecular weight: 35,000), dissolved with 15 parts of dichloromethane, added 10 parts of low-molecular-weight heparin sodium powder, stirred evenly and injected into a polytetrafluoroethylene mold, controlled the airflow speed, so that Dichloromethane evaporates. After being completely dried, the diaphragm containing low molecular weight heparin sodium was removed from the polytetrafluoroethylene mold, kept in a vacuum oven at room temperature for 48 hours to completely remove the solvent, and obtained a thickness of 0.8 mm and 10 nanometer pores. The membranous medicament of structure is punched into the medicament of the present invention ( figure 1 Shown in A), wherein the mass ratio of low molecular weight heparin sodium to carrier lactic acid-glycolic acid copolymer is 1:1. During use, this low-molecular-weight heparin sustained-release system preparation was sterilized by fumigation with ethylene oxide for 2...
Embodiment 2
[0022]According to the method and steps of Example 1, but using 2 parts of poly DL-lactic acid (PDLLA) (molecular weight: 13,000), 15 parts of dichloromethane and 10 parts of enoxaparin, a pore structure with a diameter of 1 mm and a pore structure of 20 microns was obtained. The drug stick was kept in a vacuum oven at room temperature for 48 hours to completely remove the solvent and then cut into a 2 mm long low molecular weight heparin sustained release system, wherein the mass ratio of enoxaparin to carrier poly DL-lactic acid was 5:1. Fumigate with ethylene oxide for 24 hours to sterilize, place for another week, and then implant in the posterior chamber of the rabbit eye in the same way as in Example 1. The results of postoperative macroscopic observation and local histopathological examination showed that the low-molecular-weight heparin sustained-release system has good intraocular biocompatibility, and enoxaparin can maintain a certain concentration in the aqueous humo...
Embodiment 3
[0024] The same method and steps as in Example 1, but using 8 parts of poly-L-lactic acid (PLLA) (molecular weight: 100,000), 16 parts of dichloromethane and 1 part of dalteparin sodium to obtain a pore structure with a diameter of 2 mm and a thickness of 1.5 mm. A low-molecular-weight heparin slow-release system with a size of 15 microns, in which the mass ratio of dalteparin sodium to carrier poly-L-lactic acid is 1:8, sterilized by fumigation with ethylene oxide for 24 hours before use, and then placed for 1 week before planting Into the back room of the rabbit's eye.
[0025] Postoperative macroscopic observation and local histopathological examination results show that the present invention has good intraocular biocompatibility, low molecular weight heparin can maintain a certain concentration in aqueous humor within 22 weeks after implantation, and after 25 weeks, the medicament of the present invention It disappears completely, and you don't need to take it out again. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com