A compound electrolyte material and its making method
A composite electrolyte and composition technology, applied in capacitor electrolytes/absorbents, chemical instruments and methods, circuits, etc., can solve problems such as poor long-term stability, easy leakage of liquid electrolytes, and shortened working life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] According to the liquid mixture A is 94wt%, the solid inorganic layered compound powder B is 6wt%, wherein the solid inorganic layered compound powder is Na-montmorillonite, its particle size is 50-500nm, and the specific surface area is 100m 2 / g;
[0056] Described liquid mixture is:
[0057] A-1) Ethanol 80wt%
[0058] A-2) a kind of inorganic salt (lithium iodide) 10wt%
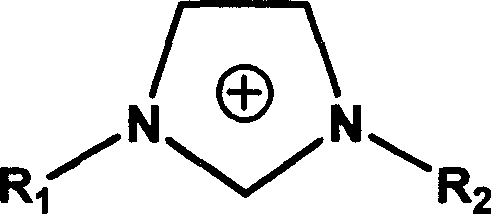
[0059] A-3) An ionic liquid (1-propyl-3-methylimidazolium iodide) 10wt%
[0060] A-4) The amount of iodine iodine and component A-2) and components
[0061] Iodine I contained in A-3) 2 / I - (Iodine) molar ratio is 1:10
[0062] The ionic liquid used in the present embodiment and the following examples, 1-propyl-3-methylimidazolium iodide salt, is the use of "Li Yongfang et al., "Chemical Bulletin", 2002 (4): 243-250) ion It is prepared by the one-step method introduced in "Progress in Liquid Research", that is, adding N-methylimidazole and n-propane iodide at a ratio of 5:4 to cyclohexane, ...
Embodiment 2
[0069] 1. Weigh according to the following components and formula:
[0070] composition
Dosage
A-1
ethanol (solvent)
20g
[0071] A-2
Lithium iodide (inorganic salt)
3g
A-3
1-Propyl-3-methylimidazolium iodide salt (ionic liquid)
3g
A-4
iodine
0.87g
A-5
4-tert-butylpyridine (additive)
3g
B
Na-montmorillonite (inorganic layered compound)
1.9g
[0072] 2. Preparation of electrolyte:
[0073] (a) Configure the liquid mixture: dissolve the weighed lithium iodide, 1-propyl 3-methylimidazolium iodide salt, iodine and additive 4-tert-butylpyridine in ethanol, and perform mechanical stirring to mix evenly to form a transparent The liquid mixture; Other steps are with embodiment 1
[0074] 3. The preparation and testing steps of the battery are the same as in Example 1. The photoelectrochemical performance test results of the battery are sho...
Embodiment 3
[0076] 1. Weigh according to the following components and formula:
[0077] composition
Dosage
A-1
ethanol (solvent)
20g
A-2
Lithium iodide (inorganic salt)
0g
A-3
1-Propyl-3-methylimidazolium iodide salt (ionic liquid)
2.5g
A-4
iodine
0.25g
A-5
4-tert-butylpyridine (additive)
2.5g
B
Na-montmorillonite (inorganic layered compound)
1.6g
[0078] 2. Preparation of electrolyte:
[0079] (a) Configure the liquid mixture: dissolve the weighed 1-propyl 3-methylimidazolium iodide salt, iodine and 4-tert-butylpyridine in ethanol, and perform mechanical stirring to mix uniformly to form a transparent liquid mixture; Step is with embodiment 1
[0080] 3. The preparation and testing steps of the battery are the same as in Example 1. The photoelectrochemical performance test results of the battery are shown in Table I.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com