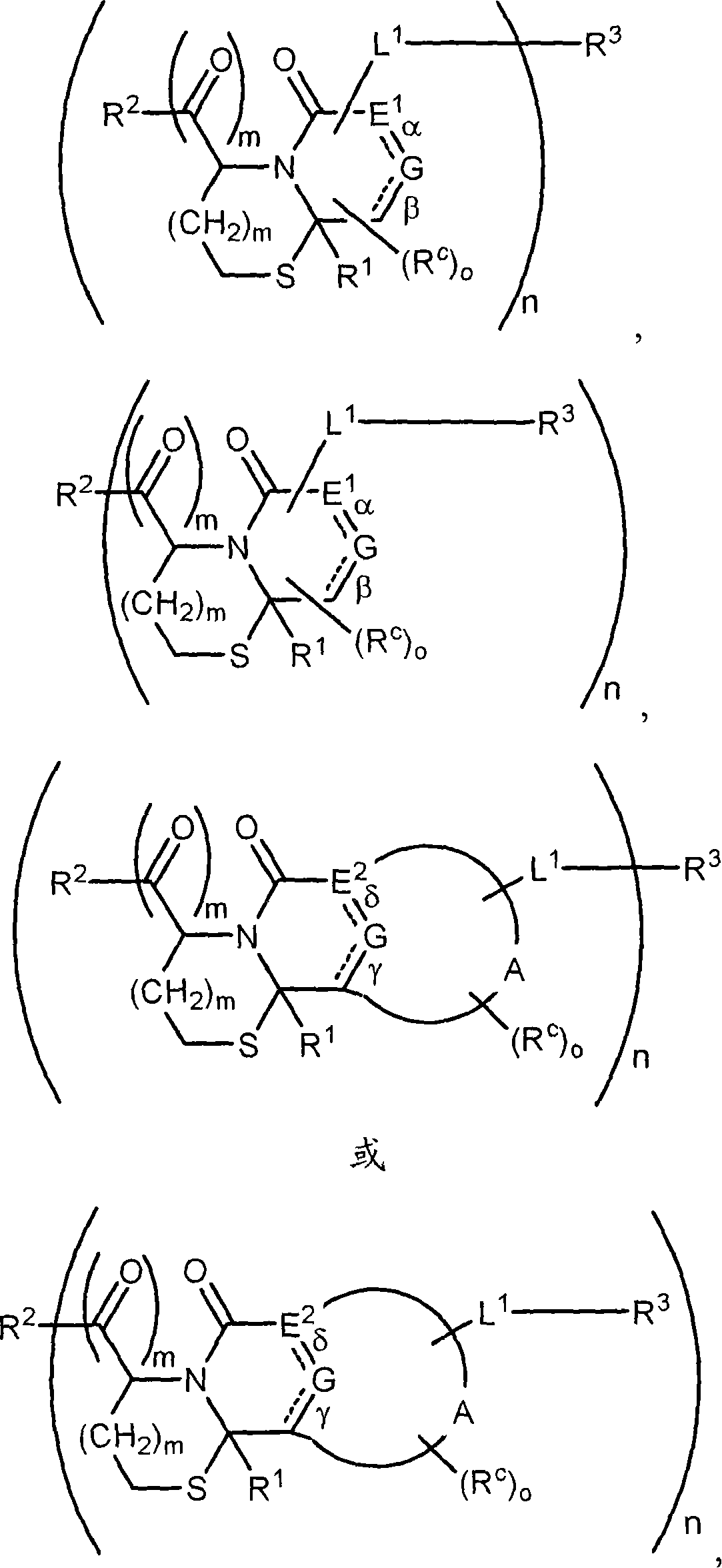
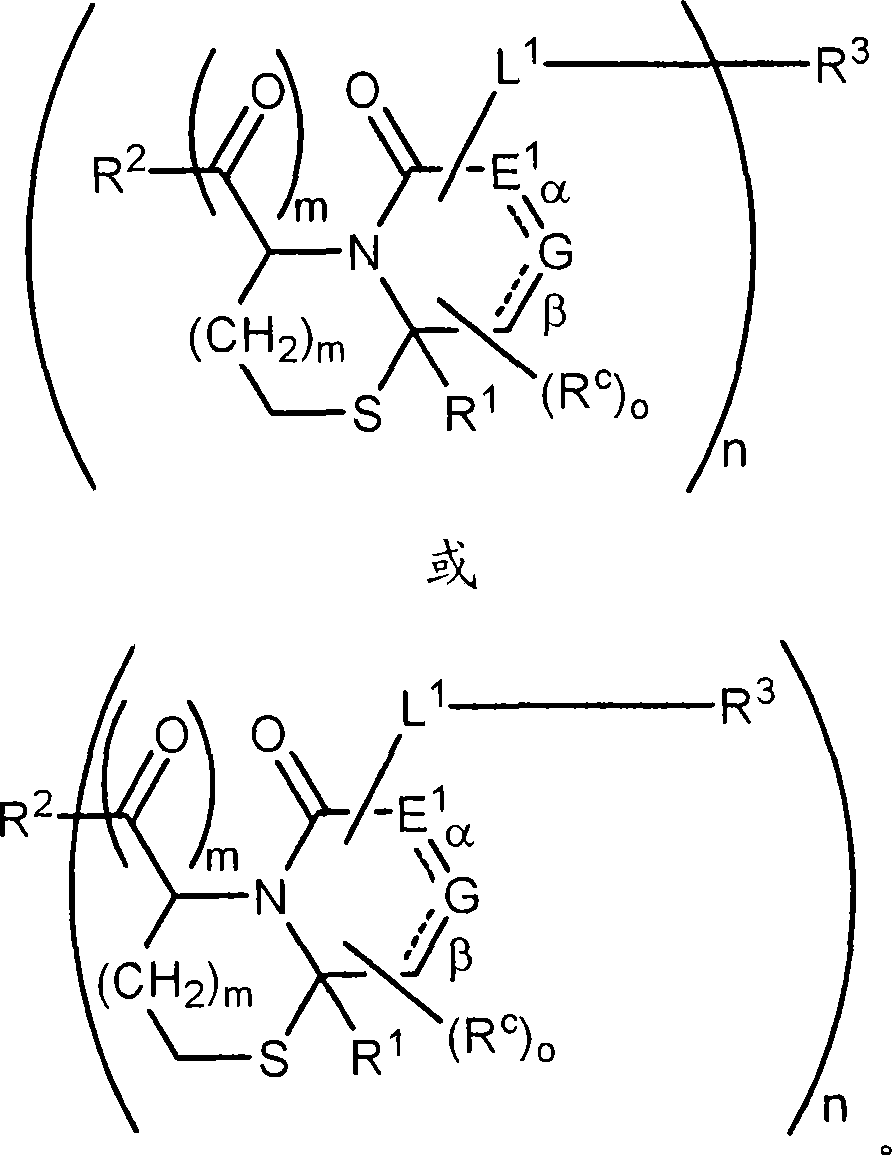
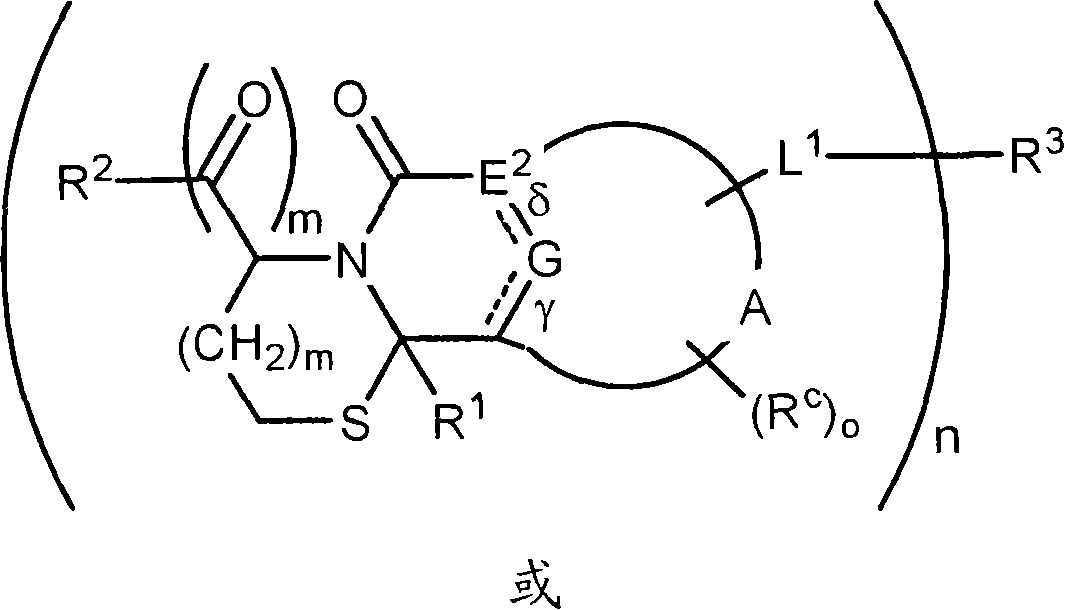
Method of conjugating aminothiol containing molecules to vehicles
A technology of carrier and alkyl, applied in the field of carrier derivatives, can solve the problems that prevent the development of biomolecules, obstacles, difficulties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0413] General experiment
[0414] NMR: Proton MR for PEG-containing molecules sees PEG singlet (3.7ppm, relative to DSS's D 2 O). 13 C NMR spectrum see PEG singlet (72.0ppm, D relative to DSS 2 O).
[0415] FTMS data were acquired on a Bruker Q-FTMS operating at a 7tesla. The instrument was calibrated externally with PEG300 / 600 solution, using the standard Francel equation. The error between the calculated mass of each calibration ion and the measured value is less than 1.0ppm. For each spectrum, 512k data points were collected with a detection scan width of 1.25 MHz (86 Da mass cutoff). Time-domain data were not processed before quantitative-mode Fourier transformation.
[0416] GC-MS data were recorded with Hewlett-Packard GC-Ms according to the following parameters:
[0417] Column: J and W DB-XLB capillary column, 30m x 0.25mm x 0.50μΜ, PN1221236.
[0418] method 1:
[0419] Injector parameters: Injector temperature = 250°C; 50:1 split ratio; Helium flow rate = 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com