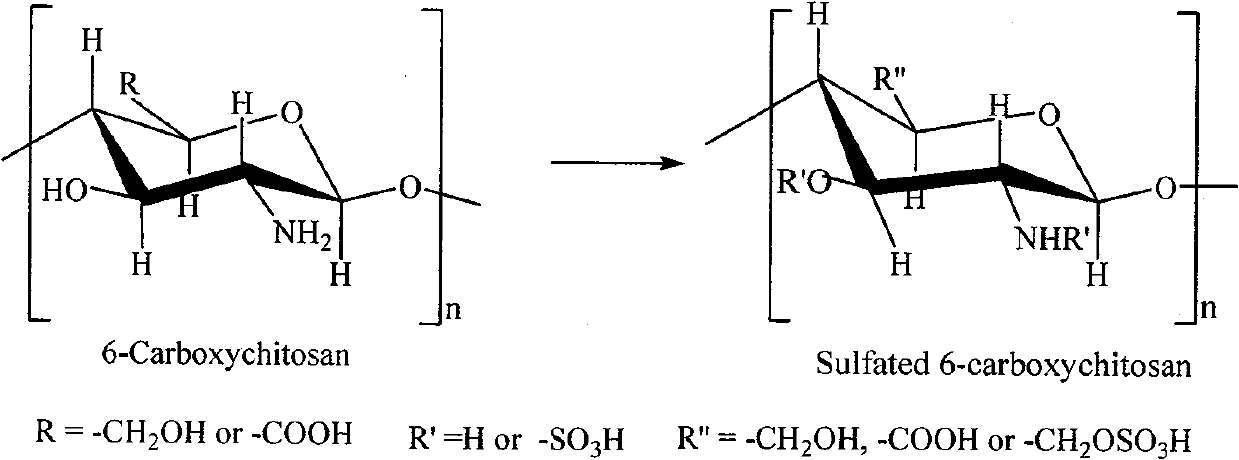
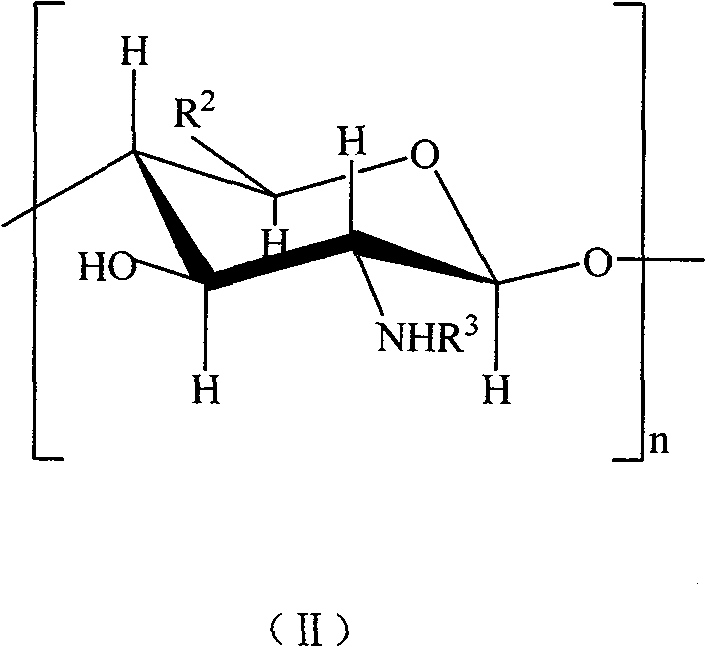
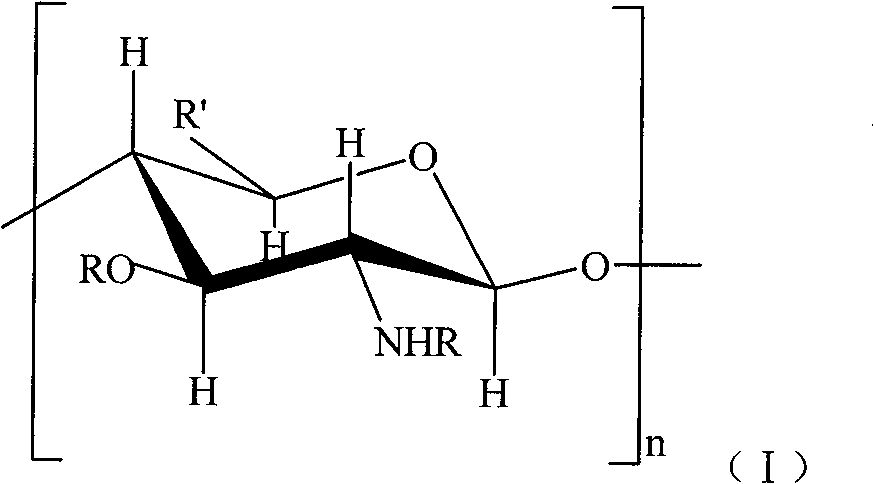
Sulfonated acylation 6-carboxyl chitosan as well as preparation method thereof
A carboxyl chitosan, sulfonated acyl technology, applied in the field of chitosan derivatives, can solve the problems of complex preparation process, poor reaction effect, difficult ion removal, etc., and achieves simple process and strong biocompatibility. Effect
Inactive Publication Date: 2010-12-08
HEBEI NORMAL UNIVERSITY OF SCIENCE AND TECHNOLOGY
View PDF2 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The reaction effect of this method is not good, and the maximum degree of carboxylation in the product does not exceed 24%.
In 1973, Horton et al. used perchloric acid to protect the amino group, using CrO 3 Preparation of 6-carboxychitosan as an oxidant, although the degree of carboxylation of the product has been greatly improved, but the preparation process is complicated and the Cr 3+ Ions are difficult to remove [Horton D, Jst E K, "Carbohydr. Res.", 1973, Vol. 29, p. 173]
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a heparin substitute expressed provided with the formula (1) of sulphonated and acidylated 6-carboxy-chitosan or the salt thereof. R expresses -H, -SO3H, or -COR<1>; R1 expresses -CH2OH, -COOH, -CH2OSO3H, or -CH2OCOR<1>; and R<1> represents C1-4 lower alkyl. The invention also relates to a preparation method of the compound. The invention has the advantages that the operation and the process are simple, and the dialysis and other tedious operation are not required for the purification of the product and the intermediate product; in addition, the reaction velocity is fast and easy to be controlled, and the sulphonated and acidylated 6-carboxy-chitosan is provided with biocompatibility and strong anticoagulant performance.
Description
technical field The invention relates to a biologically active chitosan derivative—sulfonated acylated 6-carboxy chitosan and a preparation method thereof. The compound has high anticoagulant performance. Background technique Chitosan is a biologically active natural alkaline polysaccharide extracted from shellfish such as shrimp and crab, and has poor blood compatibility. In 1971, Whistler et al. used N 2 o 4 6-carboxychitosan was prepared by reacting gas directly with dry chitosan or by dispersing chitosan in carbon tetrachloride [Whister RL, Kosik M, "Biochem.Biophys.", 1971, Vol. 142, No. 106 pages]: The reaction effect of this method is not good, and the highest degree of carboxylation in the product is no more than 24%. In 1973, Horton et al. used perchloric acid to protect the amino group, using CrO 3 Preparation of 6-carboxychitosan as an oxidant, although the degree of carboxylation of the product has been greatly improved, but the preparation process is com...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C08B37/08C08K5/42
Inventor 周永国杨越冬侯文龙
Owner HEBEI NORMAL UNIVERSITY OF SCIENCE AND TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com