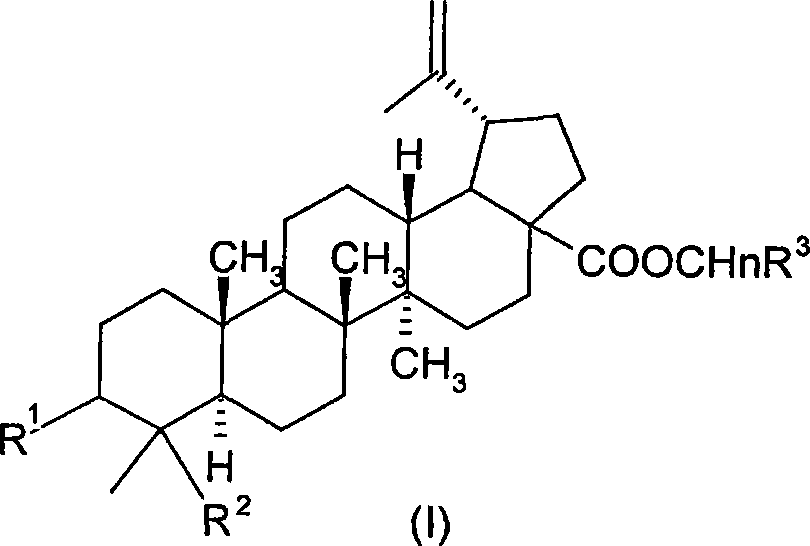
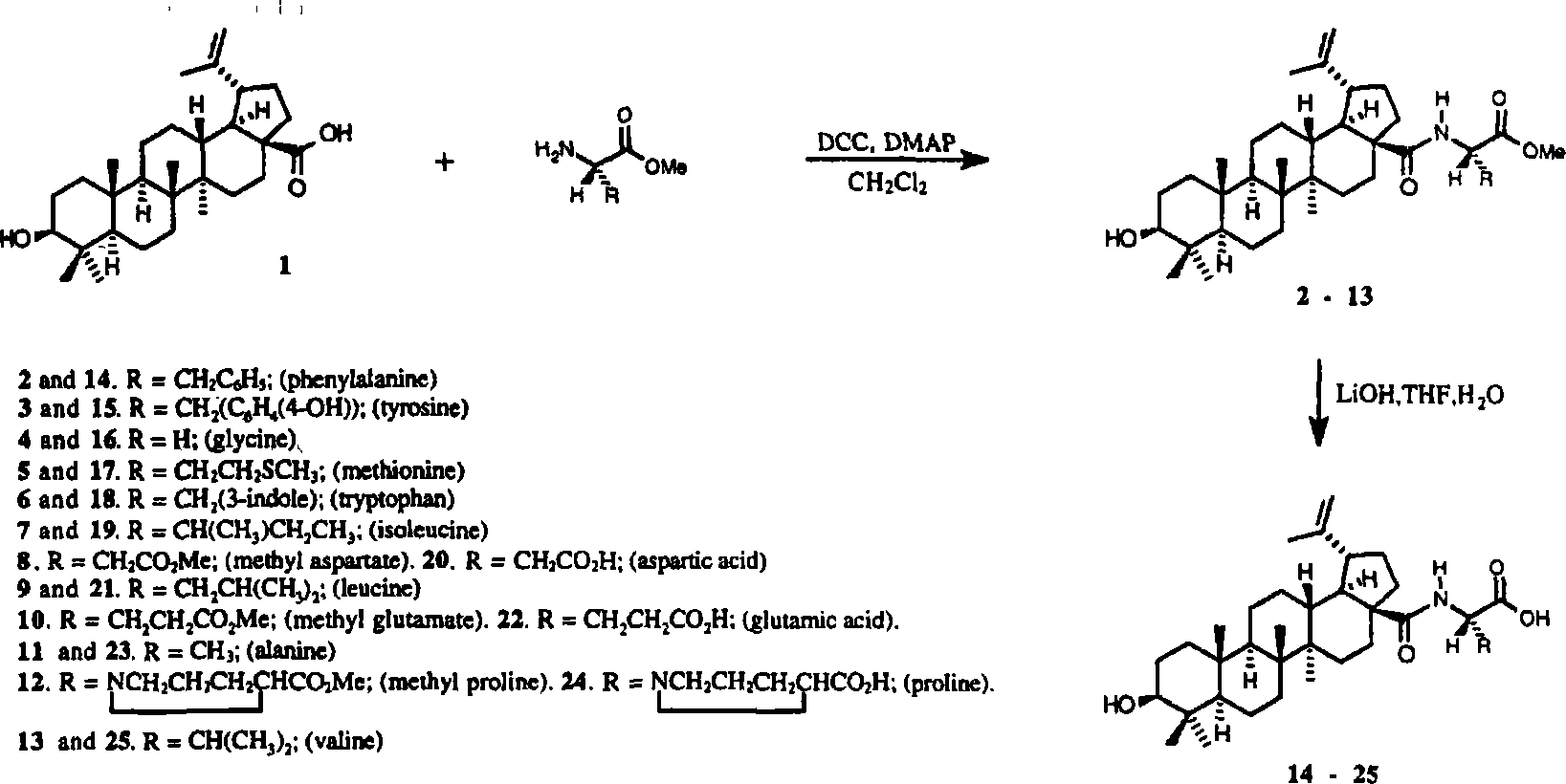
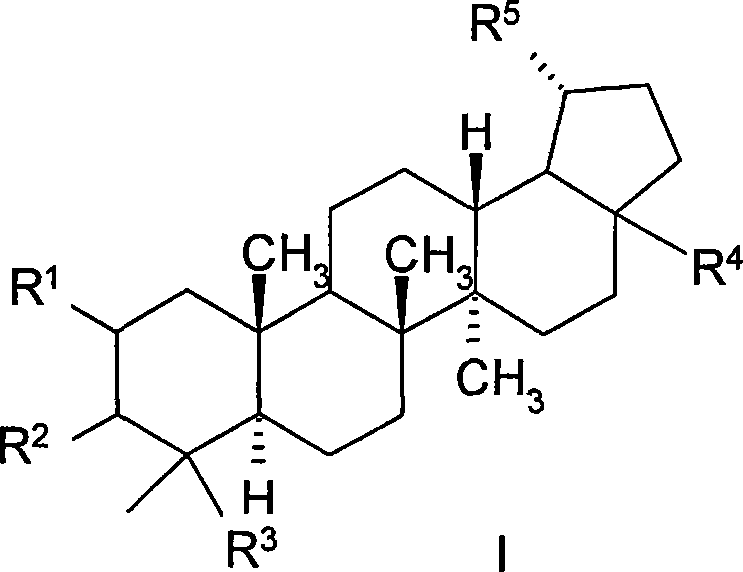
23-hydroxy betulinic acid derivative, preparation method, preparation and use thereof
A technology of betulinic acid and derivatives, applied in the field of natural medicine and medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0210] 3,23-O-Diacetyl betulinic acid (X3)
[0211] Dissolve 23-hydroxybetulinic acid (0.12g, 0.25mmol) in 5ml of pyridine in a 10ml eggplant-shaped flask, add acetic anhydride (0.15ml, 1.5mmol) dropwise, and stir overnight at room temperature. Dilute with 10ml of ethyl acetate, wash with 9% hydrochloric acid to pH 4-5, wash with saturated brine (5ml×3), dry over anhydrous magnesium sulfate, filter, and recrystallize from ethyl acetate to obtain 0.136g of light yellow powdery solid. The rate is 98%.
[0212]ESI-MS: 579[M+Na] +
[0213] 1 HNMR (300Hz, CDCl 3 ), δ (ppm): 0.80 (s, 3H, CH 3 ), 0.88 (s, 3H, CH 3 ), 0.93 (s, 3H, CH 3 ), 0.97 (s, 3H, CH 3 ), 1.69 (s, 3H, CH 3 ), 2.01 (s, 3H, CH 3 ), 2.06(s, 3H, CH 3 ), 3.00 (m, 1H, C 19 -H), 3.68~3.70(d, 1H, 23-H), 3.83~3.85(d, 1H, 23-H), 4.61(s, 1H, 29-H), 4.74(s, 1H, 29-H ), 4.75~4.78(m, 1H, 3-H)
Embodiment 2
[0215] 3,23-O-Diacetyl-28-betulinoyl chloride (BYA-2)
[0216] Dissolve 3,23-O-diacetyl-28-acid-betulinic acid (0.1g, 0.18mmol) in 5ml of refined and dried dichloromethane in a 10ml eggplant-shaped bottle, add 0.1ml of oxalyl chloride dropwise, and stir at room temperature for 4h , the dichloromethane was distilled off. Refined and dried dichloromethane (5ml×3) was added, dissolved and evaporated. Finally, 0.1 g of a light yellow glassy solid was obtained.
Embodiment 3
[0218] 3,23-Diacetyl-28-methyl betulinate (SZ1)
[0219] Dissolve BYA-2 (0.1 g, 0.17 mmol) prepared by the method in Example 2 in a 10 ml eggplant-shaped flask with 5 ml of refined and dried dichloromethane, add 5 ml of methanol dropwise, and stir at room temperature for 6 h. Wash with saturated brine (5ml×3), dry over anhydrous sodium sulfate, filter, and concentrate the filtrate to make sand. Column chromatography [petroleum ether (60°C-90°C): ethyl acetate = 12:1 (v:v)] gave 0.092 g of a white powdery solid, with a total yield of 90.0%, and mp 110-112°C.
[0220] ESI-MS: 593[M+Na] +
[0221] 1 HNMR (300Hz, CDCl 3 ), δ (ppm): 0.78 (s, 3H, CH 3 ), 0.83 (s, 3H, CH 3 ), 0.87 (s, 3H, CH 3 ), 0.89 (s, 3H, CH 3 ), 1.68 (s, 3H, CH 3 ), 1.99 (s, 3H, CH 3 ), 2.04(s, 3H, CH 3 ), 2.94~3.01 (m, 1H, C 19 -H), 3.64(s, 3H, CH 3 -O), 3.59~3.68(d, 1H, 23-H), 3.79~3.83(d, 1H, 23-H), 4.57(s, 1H, 29-H), 4.71(s, 1H, 29-H ), 4.71~4.77(m, 1H, 3-H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com