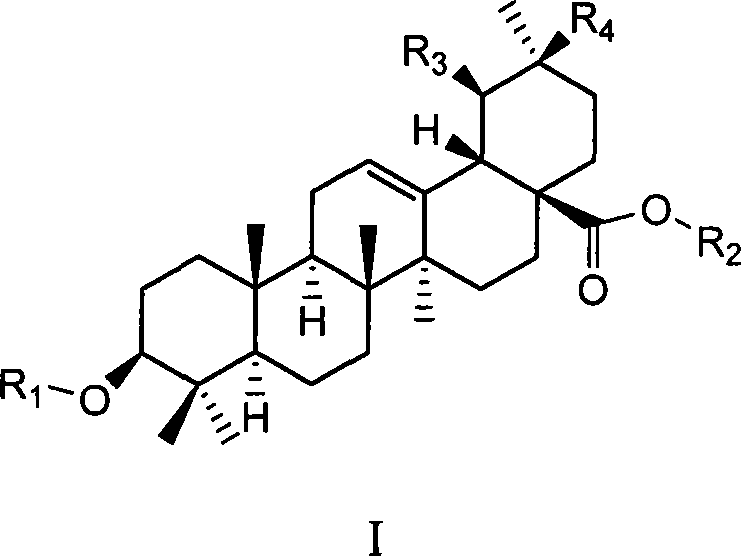
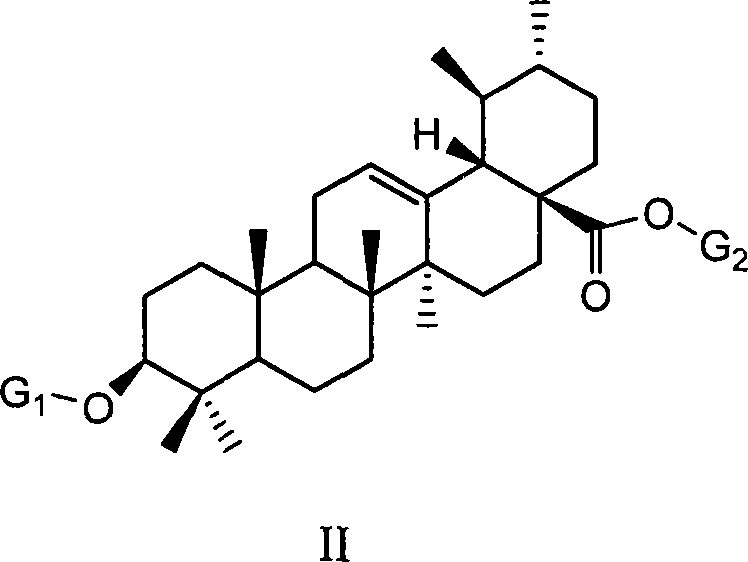
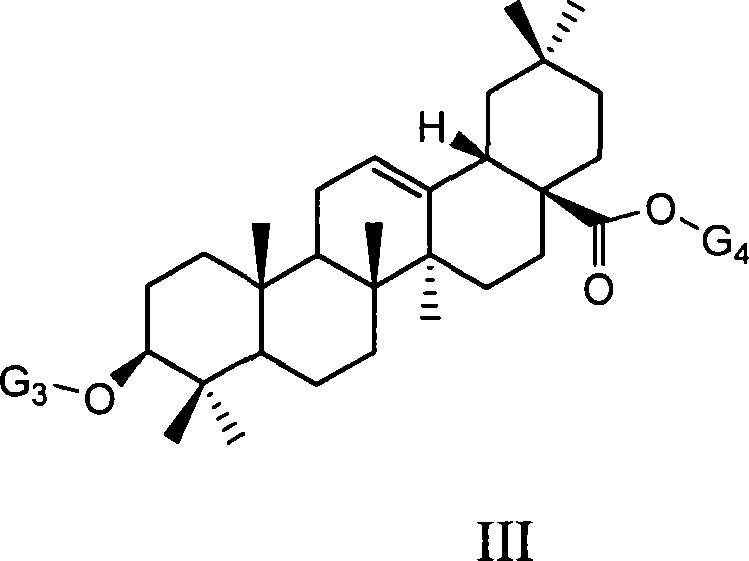
Uses of ursolic acid saponin and oleanolic acid saponin in preparing medicine for increasing white blood cell and/or blood platelet
A technology of oleanolic acid saponin and ursolic acid saponin, which is applied in the application field of preparing white blood cell and/or platelet-increasing drugs, can solve the application of ursolic acid saponin, white blood cell and/or platelet-increasing drugs that have not been seen Issues such as reporting, difficulty in conversion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Preparation of 3-O-(α-L-arabinopyranose) ursolic acid-28-O-(α-L-arabinopyranose) ester:
[0060] Ursolic acid 1g (2.2mmol) in 20ml dry CH 2 Cl 2 solution, N 2 Under atmosphere, with arabinose glycosylation donor 2,3,4-tri-O-benzoyl-β-L-arabinosyl trichloroacetimidate 3.4g (5.7mmol, 2.6eq) ( The synthesis method of this compound refers to J.Org.Chem.1999, 64, 7265-7266), after mixing 1.5 g of powdered 4 angstrom molecular sieves, trimethylsilyl trifluoromethanesulfonate TMSOTf (0.03 ml, 0.15eq), the reaction was gradually warmed to room temperature, stirred overnight, and a small amount of Et was added 3 N (0.3ml) quenched the reaction, filtered, and after the filtrate was concentrated, silica gel column chromatography was eluted with petroleum ether / ethyl acetate system (4 / 1-2 / 1) to obtain 1.47g (1.1 mmol), it was dissolved in methanol / dichloromethane (2 / 1, 30ml) and 378mgNaOMe (7mmol, 6.4eq) was added, reacted at room temperature for 4 hours, acidic resi...
Embodiment 2
[0066] Example 2: Preparation of 3-O-(α-L-rhamnopyranose) ursolic acid-28-O-(α-L-rhamnopyranose) ester:
[0067] Similar to the method of Example 1, 2,3,4-tri-benzoyl-β-L-rhamnopyranosyl trichloroacetimidate is the glycosylation donor, and 3-O-(α -L-rhamnopyranosyl)ursolic acid-28-O-(α-L-rhamnopyranosyl)ester. [ α ] D 20 = - 11.8 ( C 0.17 , DMF )
[0068] 1 HNMR (600MHz, d 5 -pyridine): δppm 6.75 (1H, s), 5.46 (1H, m), 5.32 (1H, s), 4.56-4, 57 (2H, m), 4.52 (1H, dd, J=9.12, 3.2Hz) , 4.47 (1H, dd, J = 8.9, 3.2Hz), 4.38 (1H, t, J = 9.3Hz), 4.29-4.34 (3H, m), 3.17 (H, dd, J = 11.8, 4.44Hz), 2.44(1H, d, J=11.3Hz), 1.81(1H, m), 1.70(3H, d, J=6Hz), 1.67(3H, d, J=6Hz), 1.17(3H, s), 1.08( 1H, m), 0.93(3H, s), 0.92(6H, s), 0.90(3H, d, J=6.4Hz), 0.88(3H, d, J=6.4Hz), 0...
Embodiment 3
[0071] Example 3: Preparation of 3-O-(β-D-glucopyranose) ursolic acid-28-O-(β-D-glucopyranose) ester:
[0072] Similar to the method of Example 1, the glycosylation donor is 2,3,4,6-tetra-O-benzoyl-α-D-glucosyl trichloroacetimidate, and 3-O-(β -D-glucopyranose)ursolic acid-28-O-(β-D-glucopyranose) ester. [ α ] D 20 = + 21.7 ( C 1.02 , MeOH )
[0073] IR(KBr)cm -1 : 3417, 2925, 1727, 1672, 1456, 1377, 1226, 1075, 1027, 896, 831
[0074] 1 HNMR (600MHz, CD 3 OD): δppm5.33 (d, J=8.3Hz, 1H, H-1″), 5.24 (t, 1H, H-12), 4.31 (d, J=7.8Hz, 1H, H-1′), 3.77-3.84(m, 2H), 3.65-3.68(m, 2H), 3.28--3.39(m, 7H), 3.16-3.18(m, 2H), 2.22(d, J=11.2Hz, 1H, H- 18), 1.12, 1.05, 0.96, 0.84, 0.83(s each, 3Heach, Me×5), 0.88(d, J=6.4Hz, 3H), 0.78(d, J=11.6Hz, 1H, H-5)
[0075] 13 CN...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com