Multiple RNA polymerase III promoter expression constructs
An RNA polymerase and expression construct technology, which is applied in the field of multi-RNA polymerase III promoter expression constructs and can solve problems such as small differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0093] Pharmaceutical compositions can be prepared as described herein, for example comprising DNA plasmid constructs expressing 2, 3, 4, or more RNA polymerase III promoters under the control of 2, 3, 4, or more Related to, e.g., sequences from one or more genes of a target pathogen (e.g., a virus such as HBV, HCV, HIV, HPV, variola virus), or other nucleic acid sequences associated with another pathogen or human, veterinary and agricultural Sequences such as the human cell receptor sequence of anthrax virus, or other gene sequences associated with mammalian, vertebrate, or invertebrate diseases or conditions such as Huntington's disease or VEGF, or associated with macular degeneration or genetically engineered for treatment Short hairpin dsRNA molecules substantially homologous to avian influenza virus or West Nile virus sequences of human, equine or avian host cells). Expression constructs may include additional promoter sequences, such as the bacteriophage T7 promoter, whe...
Embodiment 1
[0130] Example 1: An expression construct based on tricistronic RNA polymerase III for producing shRNAs that reduce hepatitis B virus RNA production and replication
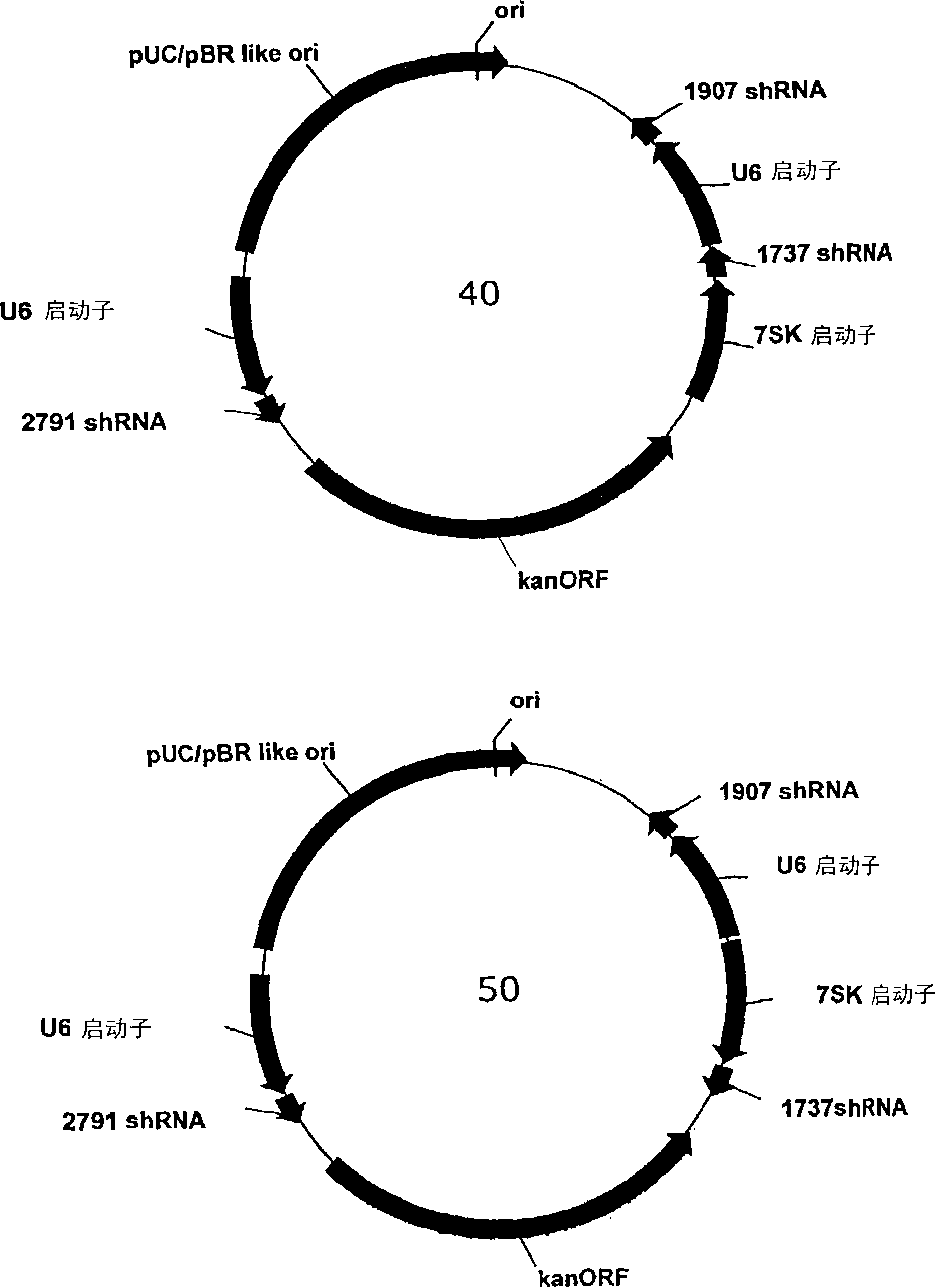
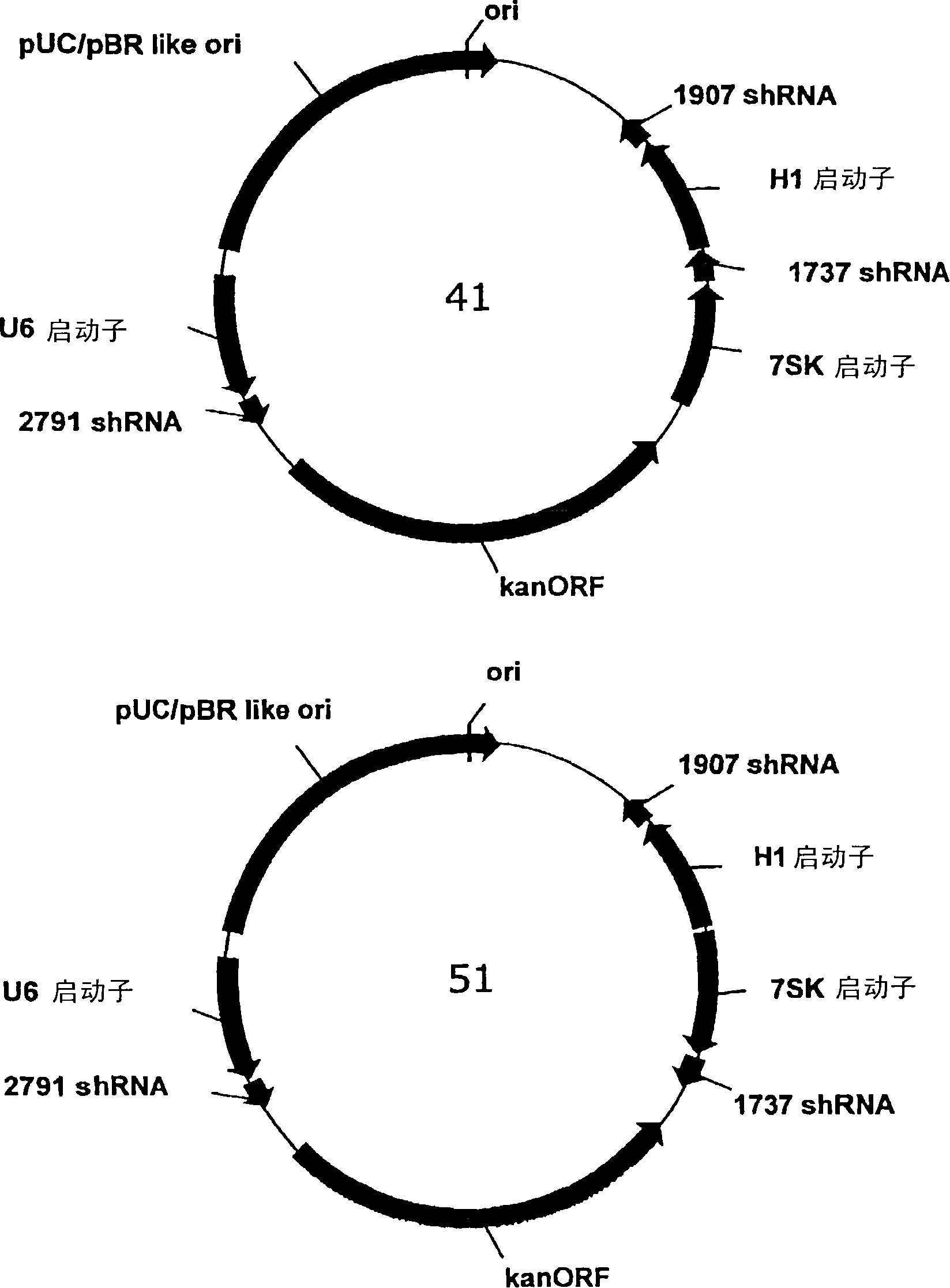
[0131] A series of plasmids was constructed to express three different shRNAs targeting hepatitis B virus under the control of three separate RNA polymerase III promoters. In the multiple cloning site of the vector, the U6 and 7SK promoter / shRNA cassettes were placed adjacent to each other, while a third promoter sequence was placed with the distant cloning site (adjacent to the kanamycin resistance gene) (Second copy of U6 promoter or H1 promoter). The 5' end of each shRNA element can be linked to each promoter's connected to the 3' end. Figure 4 The sequence of each promoter element is given. Figure 5Three shRNA sequences derived from conserved regions of HBV and placed into tricistronic vectors called 2791, 1907 and 1737 are shown. Each shRNA starts with a 21 bp HBV sequence, followed by a 9 base loop ele...
Embodiment 2
[0137] Example 2: Expression constructs based on four RNA polymerase III promoters for producing shRNAs that reduce hepatitis B virus RNA production and replication
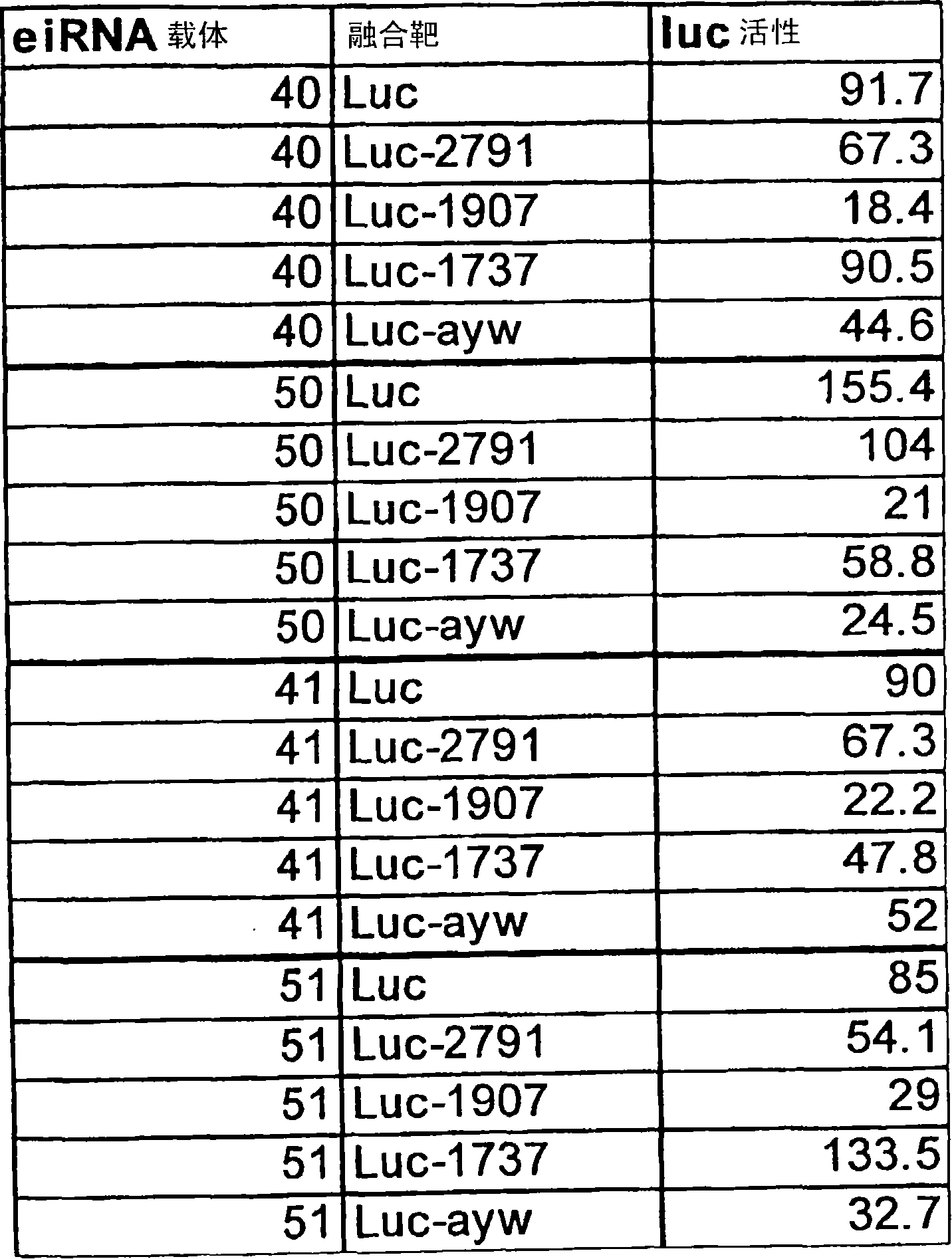
[0138] Figure 9is a schematic diagram of vector pHB4 containing 4 polymerase III promoter-shRNA cassettes. Labels in the figure illustrate the promoter name, the associated shRNA (targeted to the HBV genome) and the direction of transcription of each cassette. Vectors were constructed using an iterative method of incrementally adding shRNA cassettes, as described above. The data in Figure 10 (a luciferase assay substantially similar to that used in Example 1) demonstrates that all four promoter / shRNA cassettes are provided with a vector and a detectable substrate (luciferin in Example 1) for silencing Enzyme fusion constructs, now including the shRNA-799 construct) are active on the intracellular target sequence. The table in Figure 10 shows three independent experiments with pHB4 and the positive control, wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com