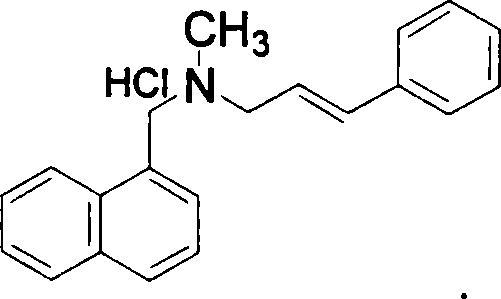
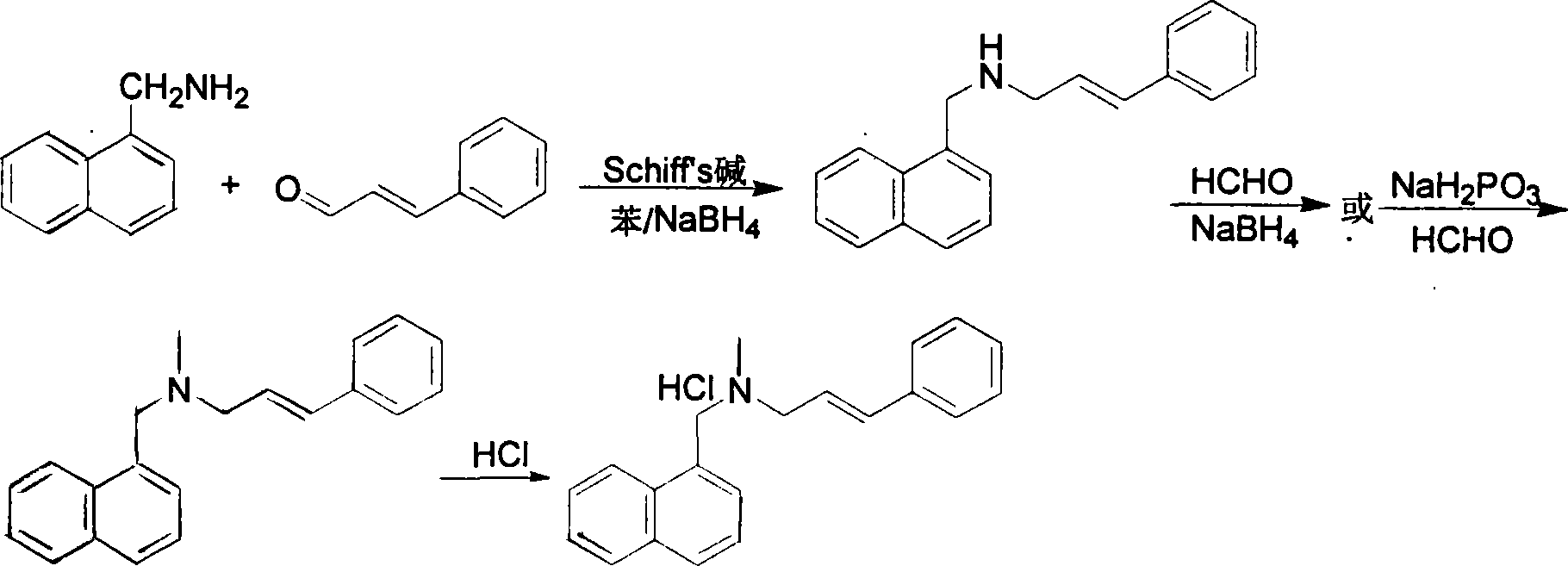
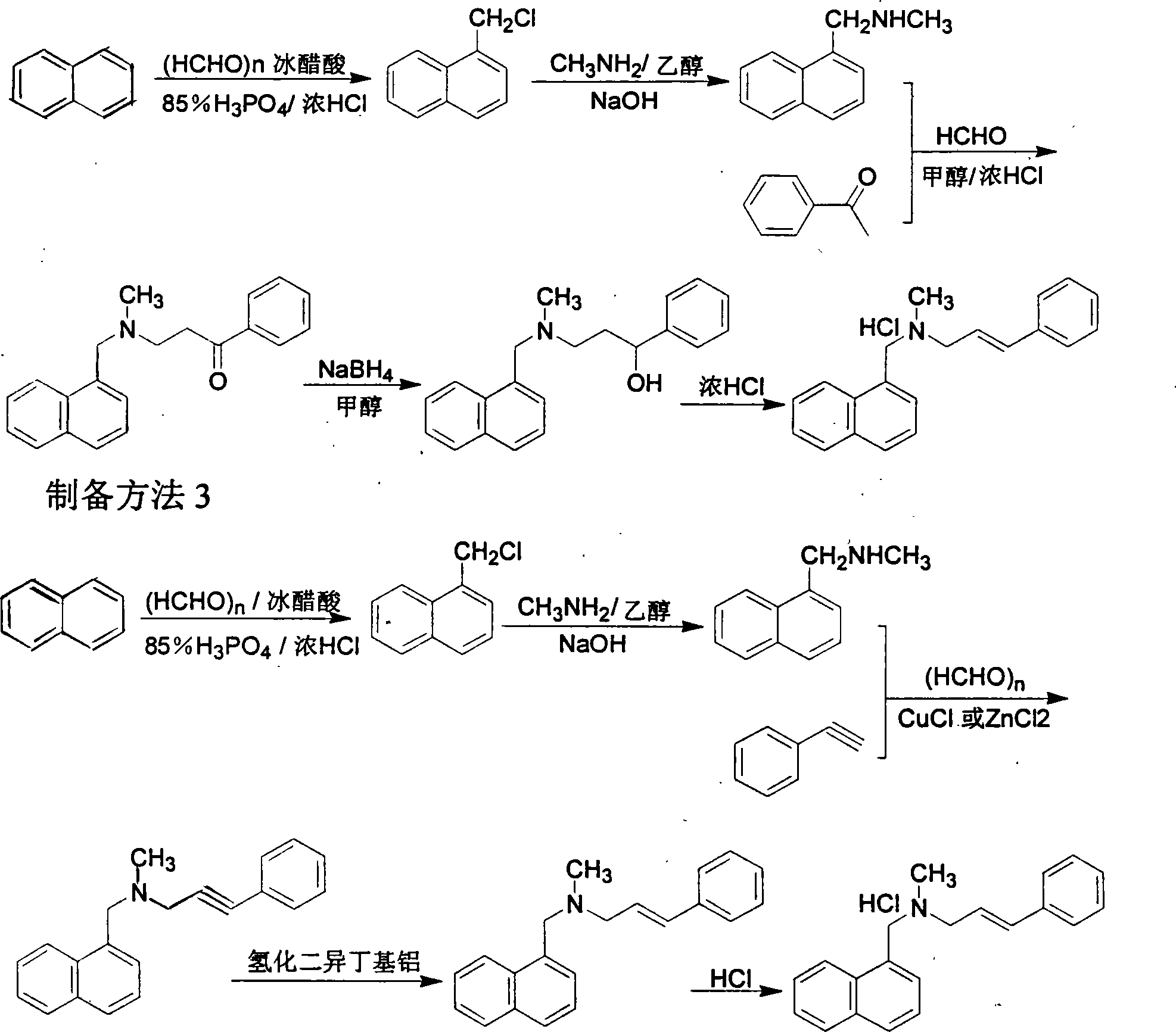
Method for preparing naftifine hydrochloride
A technology of naftifine hydrochloride and naphthalene methylamine hydrochloride, which is applied in the field of medicine, can solve problems such as poor product quality and difficult purification, and achieve the effects of low cost, simplified operation process, and industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 1.2L methyl tert-butyl ether to a 2L four-necked bottle equipped with a thermometer and mechanical stirring, and add 74.2g (0.36mol) of N-methyl-1-naphthylamine hydrochloride, 123.7g (0.90 mol) anhydrous potassium carbonate and 60g (0.1mol) PEG-600, heat the liquid temperature to reflux, stir and react for 1h, then start to add 60g (0.39mol) of cinnamyl chloride dropwise, the dropwise addition is completed within 4-6h, continue The reaction was stirred under reflux for 8 hours, followed by TLC until N-methyl-1-naphthylmethylamine disappeared, and the reaction was completed. This step can effectively control the generation of impurities in the reaction. Cool down to liquid temperature less than 20°C, filter, wash the filter cake with 50ml of methyl tert-butyl ether, combine the filtrate, add 200ml of hydrogen chloride saturated ethyl acetate solution (mass content is about 8%) dropwise under stirring, stir for a while , until a white solid was precipitated, and the d...
Embodiment 2
[0038] According to the method of embodiment 1, with diethyl ether as reaction solvent, carry out synthesis, obtain naftifine hydrochloride product 97.9g after recrystallization, yield: 84.1%, content ≥ 98% (HPLC normalization method).
[0039] The two comparative examples given below are to use the reaction solvent reported in the literature, adopt the preparation method provided by the invention to produce naftifine hydrochloride, the purpose is to prove the difference and the advantage of the preparation method provided by the invention and the preparation method reported in the literature before.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com