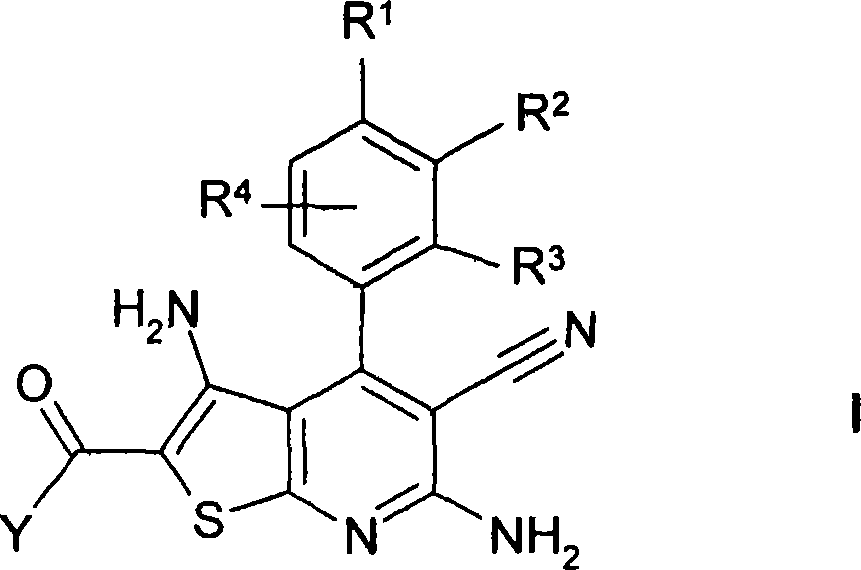
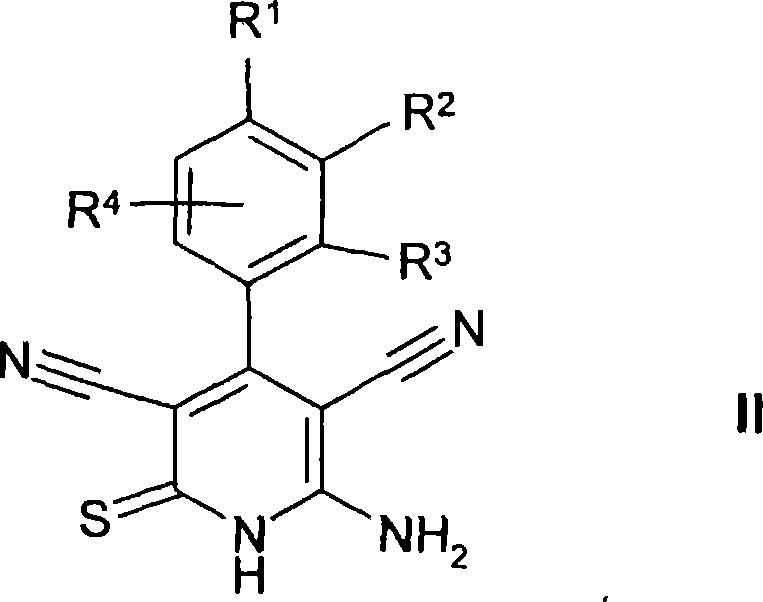
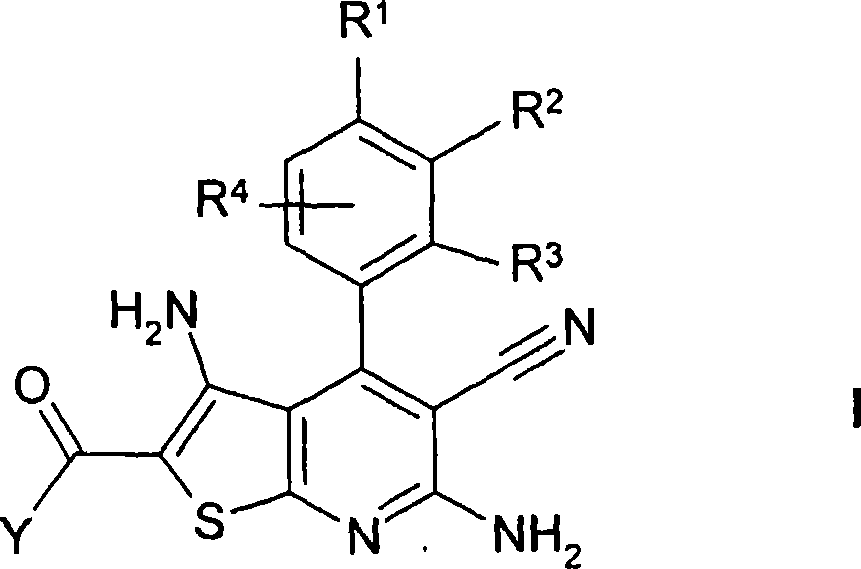
Thienopyridines
A technology of compounds and mixtures, applied in the field of treating diseases where HSP90 works, treating HSP90-induced diseases, and compounds that can be used as drugs, can solve the problems of wild-type loss of function, misregulation of molecular functions and physiological functions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0330] Prepare R 2 A general reaction scheme for compounds of formula I referring to acylated amino groups:
[0331]
[0332] Preparation of 2-aminocarbonyl-3,6-diamino-5-cyano-4-[4-methoxy-3-(3-trifluoromethylbenzoylamino)phenyl]thieno[2,3 -b] pyridine ("A1")
[0333] 1.1 10.9g of cyanothioacetamide and 9ml of 4-methylmorpholine were added to a solution of 10g of 4-methoxy-3-nitrobenzaldehyde in 100ml of ethanol, and the mixture was stirred at room temperature for 48 hours. 10% HCl was added until the pH was 5, and the mixture was stirred for a further 16 hours. The precipitated material was separated, washed with ethanol and n-heptane, and dried to obtain 9.6 g of 6-amino-3,5-dicyano-4-(4-methoxy-3-nitrophenyl)-2-sulfur Substituent-1,2-dihydropyridine ("1").
[0334]
[0335] 1.2 Add 1 equivalent of KOH dissolved in water to 30.7g of "1" in 100ml of DMF solution. Then 8.8 g of 2-chloroacetamide were added and the mixture was stirred for a further 1 hour. An addit...
Embodiment 2
[0357] Under standard conditions,
[0358] Ester hydrolysis of "A4a" gave the compound 2-aminocarbonyl-3,6-diamino-5-cyano-4-[4-methoxy-3-(4-carboxybutyrylamino)phenyl]thieno [2,3-b]pyridine ("A4"), M+H + 469.49;
[0359] Ester hydrolysis of "A6a" affords the compound 2-aminocarbonyl-3,6-diamino-5-cyano-4-[4-methoxy-3-(4-carboxybenzoylamino)phenyl]thiophene And[2,3-b]pyridine ("A6"), M+H + 503.51;
[0360] Ester hydrolysis of "A7a" affords the compound 2-aminocarbonyl-3,6-diamino-5-cyano-4-[4-methoxy-3-(2-carboxymethoxyacetamido)phenyl] Thieno[2,3-b]pyridine ("A7"), M+H + 471.46;
Embodiment 3
[0362] Add 82 mg of N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (DAPECI) and 47 μl of 4-methylmorpholine to 55 mg of BOC-glycine (BOC-Gly-OH) and 48 mg of 1-hydroxybenzotriazole hydrate was dissolved in 1 ml of DMF, and the mixture was stirred at room temperature for 1 hour. 100 mg of "3" was added and the mixture was stirred for a further 16 hours. The mixture was stirred and added to 10 ml of water, the precipitate was separated and washed with water to give
[0363] 2-aminocarbonyl-3,6-diamino-5-cyano-4-(4-methoxy-3-{2-[(tert-butoxycarbonyl)amino]acetamido}phenyl)thieno[2 , 3-b] pyridine ("A10"),
[0364]
[0365] Similarly, "3" is reacted with the following compound, which is
[0366] BOC-β-Ala-OH (BOC-β-alanine),
[0367] BOC-GABA-OH (BOC-γ-aminobutyric acid),
[0368] 1H-indole-7-carboxylic acid,
[0369] BOC-His-OH (BOC-Histidine),
[0370] BOC-Asn-OH (BOC-Asn-OH),
[0371] N-(2-carbamoylacetyl)glycine,
[0372] 1H-indazole-7-carboxylic acid,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com