Polymorphic forms of 1-'4-(5-cyanoindol-3-yl)butyl-4-(2-carbamoylbenzofuran-5-yl)piperazine hydrochloride
A technology of cyanindole and benzofuran, which is applied in the field of treatment of medical diseases and can solve the problems of incomparability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
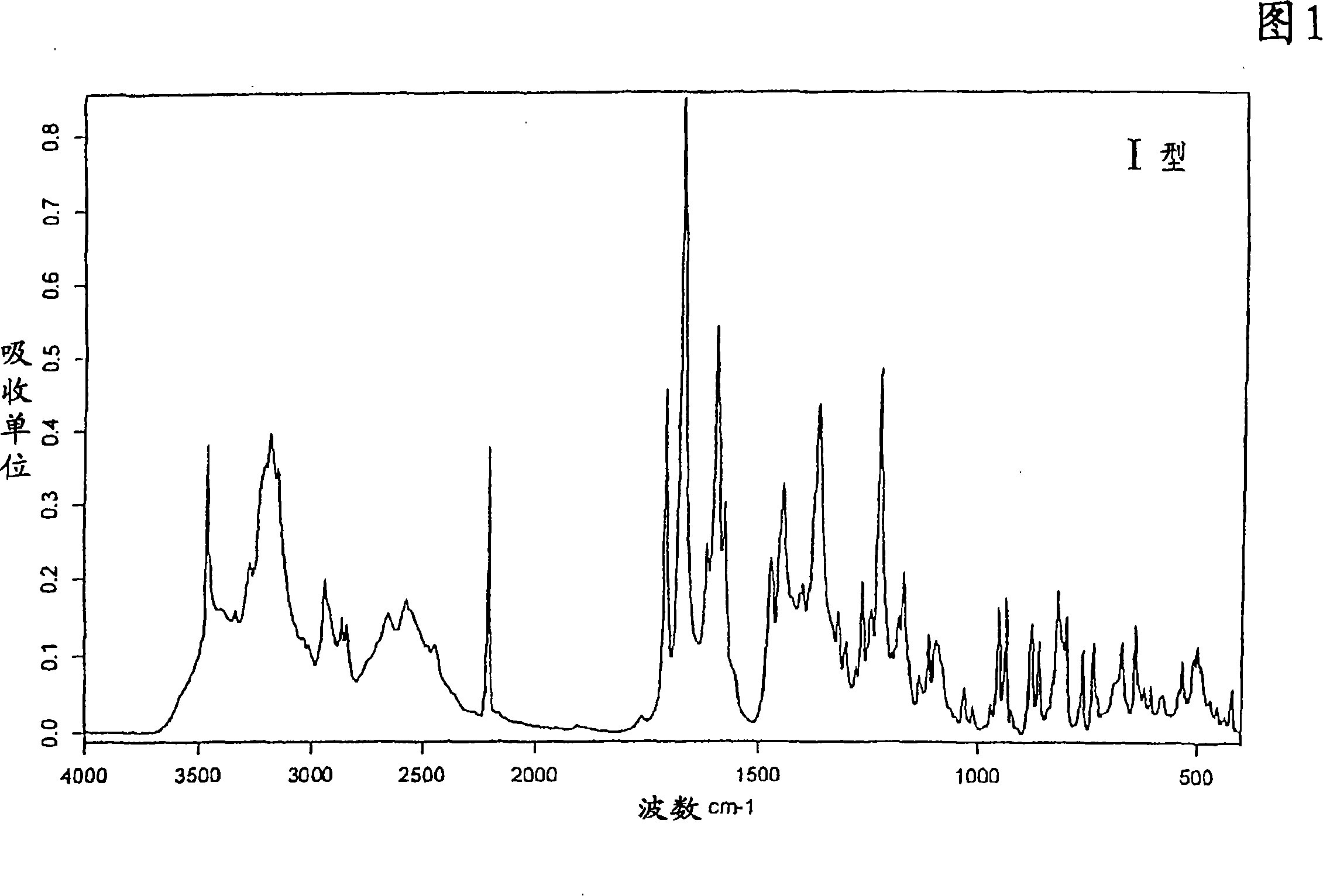
[0243] Preparation of Type I 1-[4-(5-Cyanoindol-3-yl)butyl]-4-(2-carbamoyl-benzofuran-5-yl)-piperazine hydrochloride
[0244] method 1:
[0245] 1 g of 1-[4-(5-cyanoindol-3-yl)butyl]-4-(2-carbamoyl-benzofuran-5-yl)-piperazine was dissolved in 80 ml of acetone. The temperature of the solution was brought to 50°C, and 0.5 ml of 1N hydrochloric acid was added to the reaction mixture. After stirring for 2 to 3 minutes, the reaction mixture was cooled to room temperature and a precipitate appeared. The precipitated crystals were filtered with suction. Drying to constant weight in vacuo at room temperature gave 1-[4-(5-cyanoindol-3-yl)butyl]-4-(2-carbamoyl-benzofuran-5-yl)-piperidine oxazine hydrochloride acetonate type I.
[0246] Method 2:
[0247] Disperse 2.25g of 1-[4-(5-cyanoindol-3-yl)butyl]-4-(2-carbamoyl-benzofuran-5-yl)-piperazine hydrochloride type III in 200ml of acetone. After stirring for 14 days, the precipitated crystals were recovered by filtration, and dried...
Embodiment 2
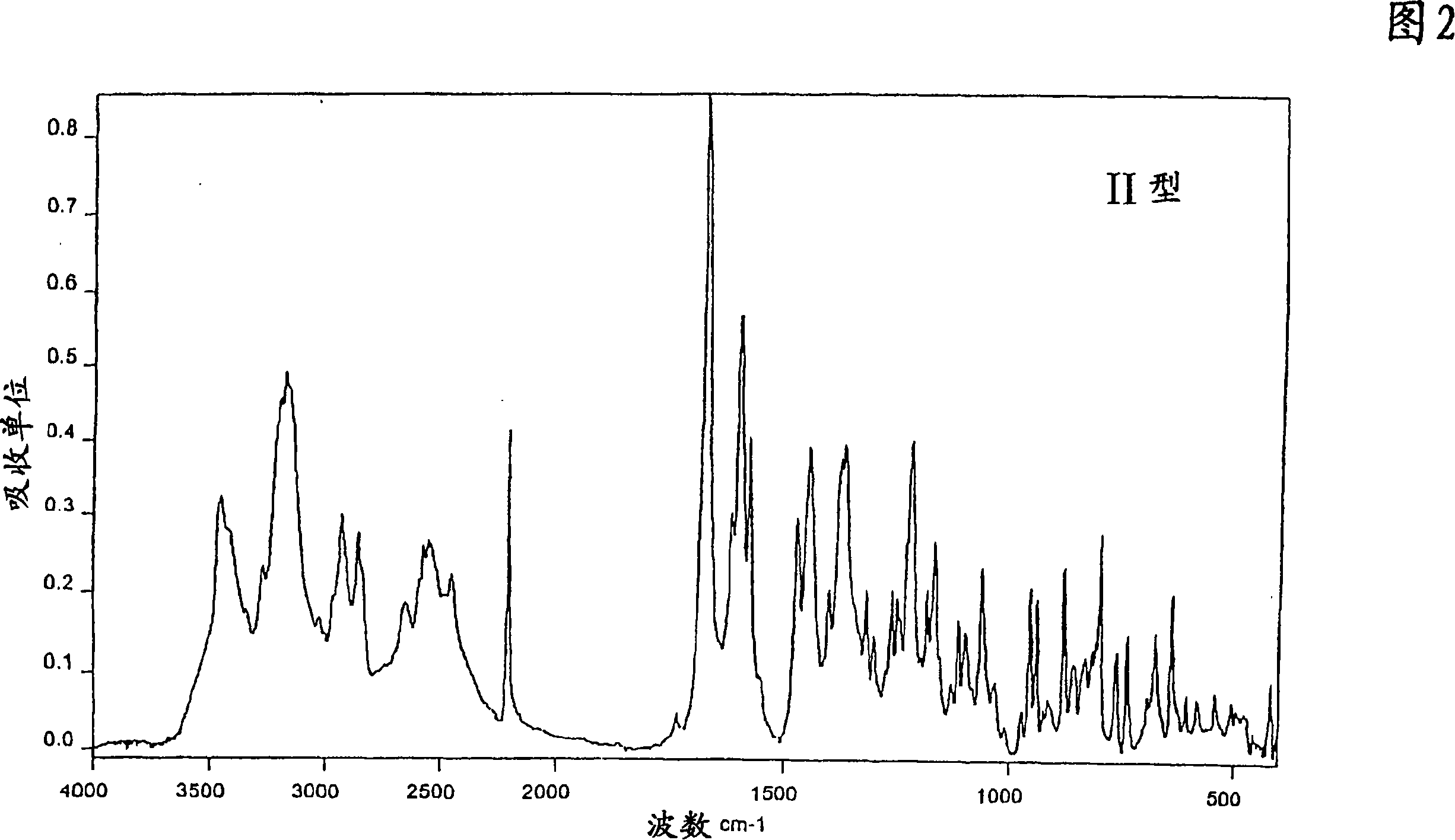
[0249] Preparation of Type II 1-[4-(5-Cyanoindol-3-yl)butyl]-4-(2-carbamoyl-benzofuran-5-yl)-piperazine hydrochloride
[0250] method 1:
[0251] Dissolve 1 g of 1-[4-(5-cyanoindol-3-yl)butyl]-4-(2-carbamoyl-benzofuran-5-yl)-piperazine in 46.6 g of tetrahydrofuran To the reaction mixture was added 2.2 g of 1N hydrochloric acid. After precipitation and stirring for 30 minutes, the precipitated crystals were subjected to suction filtration. Drying to constant weight in vacuo at room temperature gave 1-[4-(5-cyanoindol-3-yl)butyl]-4-(2-carbamoyl-benzofuran-5-yl)-piperidine Monosolvate Form II of oxazine hydrochloride with tetrahydrofuran, which exhibits the IR absorption spectrum of FIG. 2 and the X-ray diffraction spectrum of FIG. 13 .
[0252] Method 2:
[0253] Disperse 3 g of 1-[4-(5-cyanoindol-3-yl)butyl]-4-(2-carbamoyl-benzofuran-5-yl)-piperazine hydrochloride type III in 400ml of tetrahydrofuran. After stirring for 20 days, the precipitated crystals were recovered by...
Embodiment 3
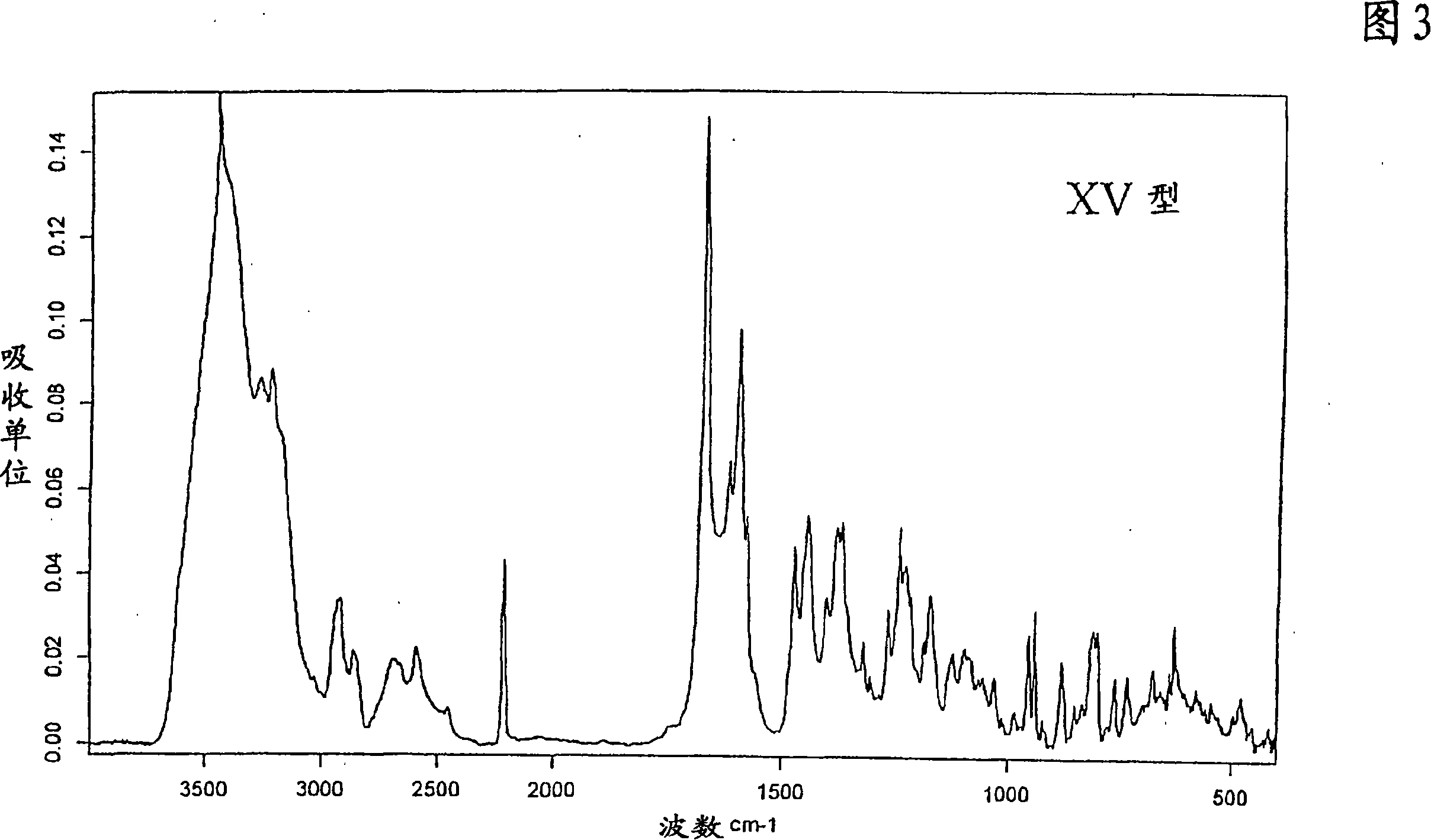
[0255] Preparation of Type XV 1-[4-(5-Cyanoindol-3-yl)butyl]-4-(2-carbamoyl-benzofuran-5-yl)-piperazine hydrochloride
[0256] To 1-[4-(5-cyanoindol-3-yl)butyl]-4-(2-carbamoyl-benzofuran-5-yl)-piperazine hydrochloride at 0 °C 10 ml of 1N hydrochloric acid was added to a solution of tetrahydrofuran (200 ml) (molar ratio of base to tetrahydrofuran = 1:48). After stirring for 30 minutes, the precipitated crystals were recovered by filtration. Drying to constant weight in vacuo at room temperature gave 1-[4-(5-cyanoindol-3-yl)butyl]-4-(2-carbamoyl-benzofuran-5-yl)-piperidine A solvate of oxazine hydrochloride with tetrahydrofuran, Form XV, which exhibits the IR absorption spectrum of FIG. 3 and the X-ray diffraction spectrum of FIG. 14 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com