Bispecific domain antibodies targeting serum albumin and GLP-1 or PYY
A serum albumin and GLP-1 technology, applied in the field of bispecific domain antibodies targeting serum albumin and GLP-1 or PYY, can solve the problem of not producing long-acting active GLP-1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0134] Preparation of immunizing antigens, polyclonal and monoclonal antibody production can be performed using any suitable technique. Various methods have been described. (See, for example, Kohler et al., Nature, 256:495-497 (1975) and Eur.J.Immunol.6:511-519 (1976); Milstein et al., Nature 266:550-552 (1977); Koprowski et al., U.S. Patent No. 4,172,124; Harlow, E. and D. Lane, 1988, Antibodies: A Laboratory Manual, (Cold Spring Harbor Laboratory: Cold Spring Harbor, NY); Current Protocols In Molecular Biology, Vol.2 (Supplement 27, Summer' 94), in Ausubel, F.M. et al. (John Wiley & Sons: New York, NY), Chapter 11, (1991).). Generally, when monoclonal antibodies are desired, hybridomas are produced by fusing appropriate cells from an immortal cell line (eg, a myeloma cell line such as SP2 / 0, P3X63Ag8.653, or heteromyeloma) with antibody-producing cells. Antibody-producing cells can be obtained from the peripheral blood or, preferably, the spleen or lymph nodes of humans, h...
Embodiment 1
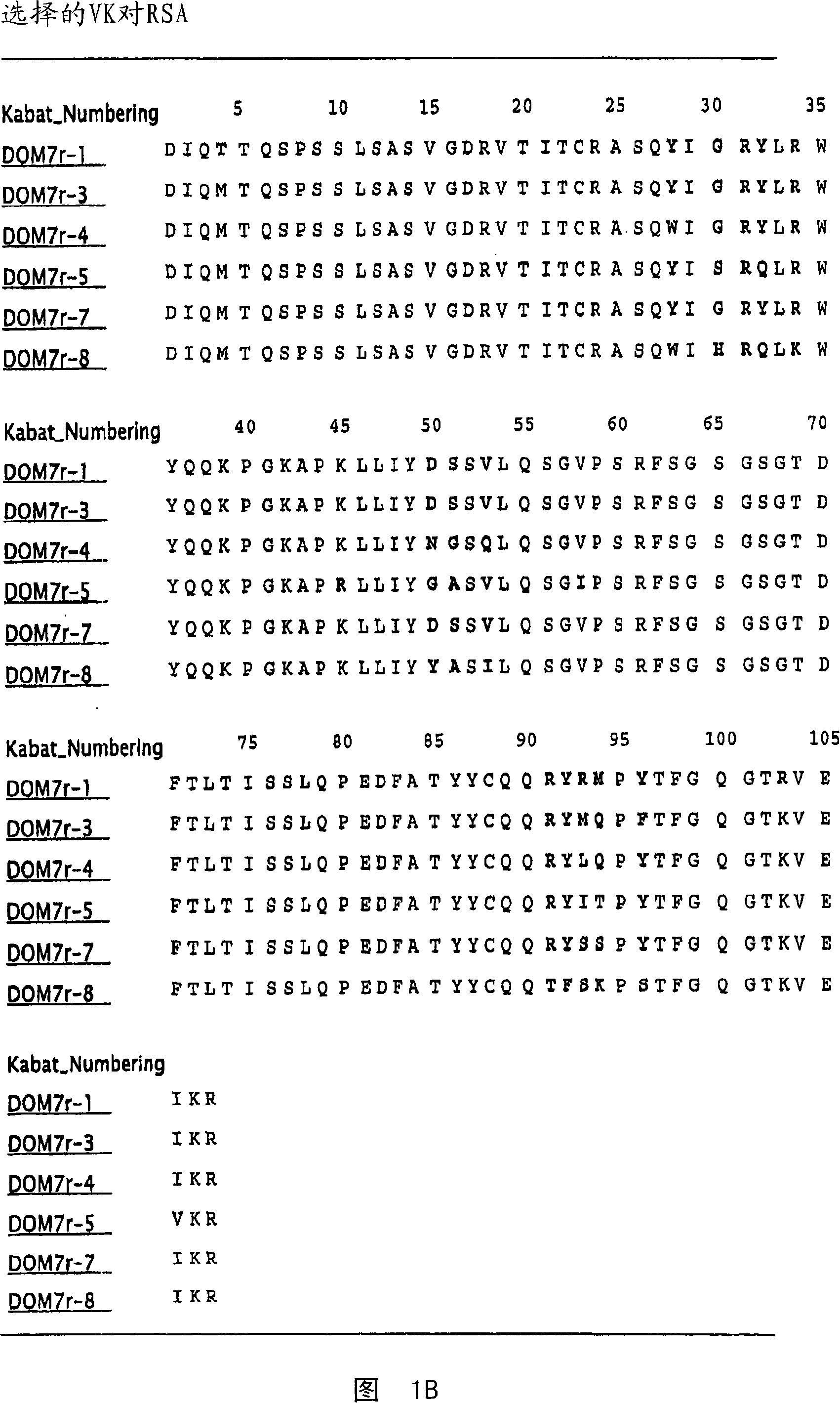
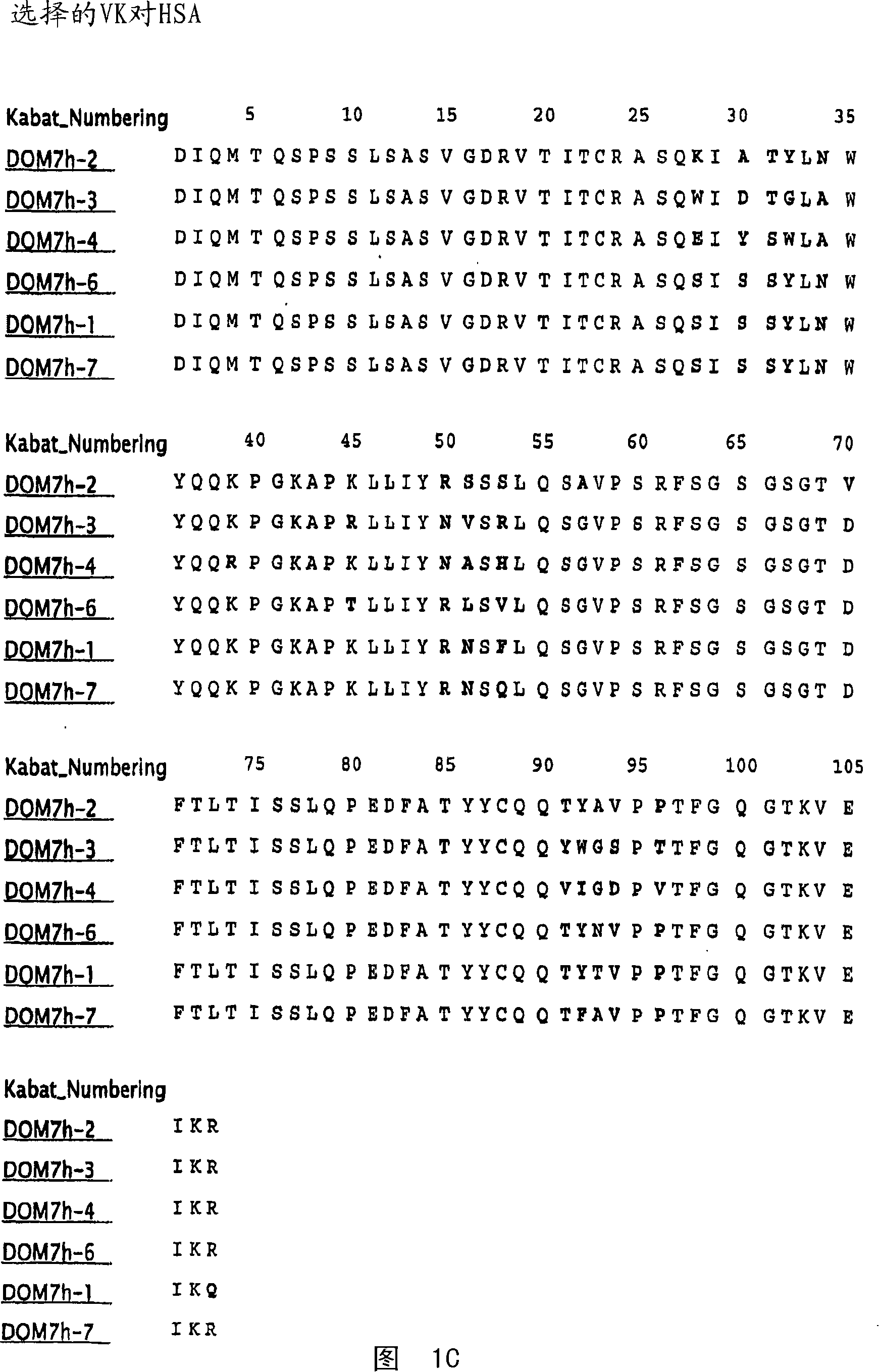
[0352] Example 1 Selection of Domain Antibodies Binding to Mouse, Rat and Human Serum Albumin
[0353] This example illustrates a method for making single domain antibodies (dAbs) directed against serum albumin. A selection of dAbs directed against mouse serum albumin (MSA), human serum albumin (HSA) and rat serum albumin (RSA) is described.
[0354] dAbs directed against mouse serum albumin were selected as described in WO2004 / 003019A2. Three human phage display antibody libraries were used. Each library is based on V H (V3-23 / DP47 and J H 4b) or V κ (o12 / o2 / DPK9 and J κ 1) Single person frame, V H and V κ Side chain diversity encoded by NNK codons introduced in the complementarity determining fragments (CDR1, CDR2 and CDR3).
[0355] Library 1 (V H )
[0356] Diversity positions are: H30, H31, H33, H35, H50, H52, H52a, H53, H55, H56, H58, H95, H97, H98
[0357] Library size: 6.2×10 9
[0358] Library 2 (V H ):
[0359] The diversity positions are: H30, H31, H3...
Embodiment 2
[0377] Example 2. Formatting Anti-Serum Albumin Antibody as a Fusion to IL-1 Receptor Antagonist (IL-1ra)
[0378] This example describes a method for making a fusion protein comprising IL-1ra and a dAb that binds serum albumin. Two fusions were formed, one with the dAb N-terminus of IL-1ra (MSA16IL1-ra) and one with the dAb C-terminus of IL-1ra (IL1-ra MSA 16). The sequence and vector of the fusions are shown in Figures 2C and 2D. A control fusion that did not bind MSA was also generated, the sequence of which is shown in Figure 2E.
[0379] KINERET (anakina, Amgen) has a short half-life of 4-6 hours and the recommended dosing regimen calls for daily injections. This regimen resulted in injection site reactions within 14-28 days in 71% of cases. Therefore, a form of human IL-1ra with a longer serum half-life would be beneficial and allow for increased efficacy and less frequent dosing. These are desirable characteristics for pharmacology.
[0380] clone
[0381] Briefly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com