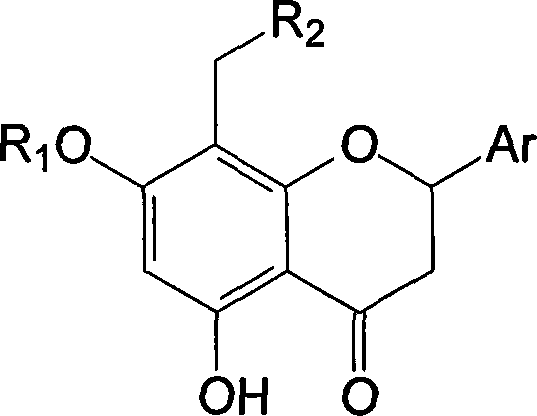
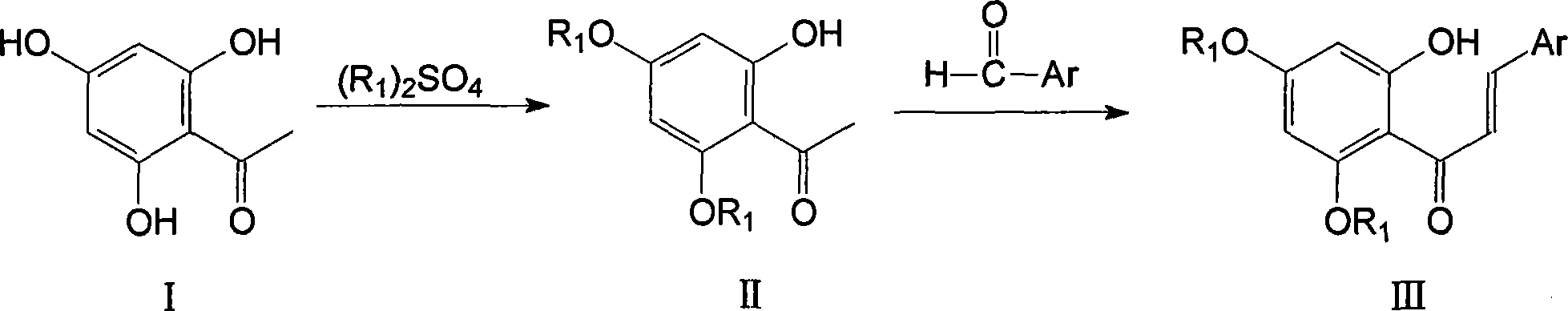
Polysubstituted flavanone derivative and its prepn and application
A derivative, the technology of amine methylene flavanone, applied in the field of synthesis of organic compounds, can solve problems such as the mechanism of anti-tumor effect that has not been studied in depth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1, 2'-hydroxyl-3'-(morpholin-1-yl-methyl)-4',6'-dimethoxy-2-chloro-chalcone (IVa):
[0023] 1.59g (4.99mmol) of compound III, 1.24g (15.30mmol) of formaldehyde aqueous solution, 1.00g (11.49mmol) of morpholine, 13ml of methanol and hydrochloric acid were added dropwise into the reaction flask, and refluxed for 2h. Cool, recover the solvent under reduced pressure, dilute with water, basify, extract with dichloromethane, wash with saturated sodium chloride, dry over anhydrous sodium sulfate, recover the solvent under reduced pressure, and separate by column chromatography to obtain 1.30 g of a yellow solid with a yield of 61%. m.p.162-164°C.
[0024] 1 HNMR (CDCl 3 ): δ8.04(d, 1H, J=15.6Hz), 7.70-7.68(m, 1H), 7.57(d, 1H, J=15.6Hz), 7.43-7.41(m, 1H), 7.30-7.28( m, 2H), 6.02(s, 1H), 3.91(s, 3H), 3.89(s, 3H), 3.71(t, 4H, J=4.4Hz), 3.67(s, 2H), 2.56(t, 4H , J=4.4Hz).
Embodiment 2
[0025] Example 2, 2'-hydroxyl-3'-(piperidin-1-yl-methyl)-4',6'-dimethoxy-2-chloro-chalcone (IVb):
[0026] Refer to Example 1 for the operation process, except that piperidine is used instead of morpholine to obtain yellow crystals with a yield of 50%, m.p.159-162°C.
[0027] 1 HNMR (CDCl 3 ): δ7.94(d, 1H, J=16.0Hz), 7.69-7.66(m, 1H), 7.38-7.36(m, 1H), 7.26-7.24(m, 2H), 7.22(d, 1H, J =16.0Hz), 5.99(s, 1H), 3.82(s, 3H), 3.81(s, 3H), 3.66(s, 2H), 2.50-2.40(m, 4H), 1.59-1.55(m, 4H) , 1.42-1.40 (m, 2H).
Embodiment 3
[0028] Example 3, 2'-hydroxyl-3'-(tetrahydropyrrol-1-yl-methyl)-4',6'-dimethoxy-2-chloro-chalcone (IVc):
[0029] Refer to Example 1 for the operation process, except that tetrahydropyrrole is used instead of morpholine to obtain yellow crystals with a yield of 50% and m.p.125-127°C.
[0030] 1 HNMR (CDCl 3 ): δ8.05(d, 1H, J=15.6Hz), 7.70-7.68(m, 1H), 7.54(d, 1H, J=15.6Hz), 7.43-7.40(m, 1H), 7.30-7.28( m, 2H), 6.02 (s, 1H), 3.94 (s, 2H), 3.93 (s, 3H), 3.91 (s, 3H), 2.86 (brs, 4H), 1.89 (brs, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com