Method for detecting n-terminal pre-BNP
A sample and antibody technology, applied in the direction of microorganism-based methods, chemical instruments and methods, biochemical equipment and methods, etc., can solve problems such as no detection, achieve good resolution, prolong survival probability, and easy survival probability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Production method of recombinant N-terminal proBNP (1-76)
[0053] 1. Cloning of recombinant N-terminal proBNP
[0054] The nucleotide sequence of N-terminal proBNP (amino acid sequence 1-76) was produced by means of genetic synthesis. For optimal expression of the gene in E. coli, the sequence was adapted to the most commonly used codons in E. coli. The oligonucleotide sequence used to generate the gene is as follows:
[0055] Pro5' (SEQ ID NO1)
[0056] 5'CCGGATCCCACCCGCTG3'
[0057] Prolhum (SEQ ID NO2):
[0058] 5'CGGGATCCCACCCGCTGGGTTCCCCGGGTTCCGCTTCCGACCTGGAAACCT
[0059] CCGGTCTGCAGOAACAGCGTAACCACCT3'
[0060] Pro2hum (SEQ ID NO3).
[0061] 5'CGGTTCCAGGGAGGTCTGTTCAACCTGCAGTTCGGACAGTTTACCCTGCAG
[0062] GTCGTTACGCTGTTCCTGC3'
[0063] Pro3hum (SEQ ID NO4).
[0064] 5'CAGACCTCCCCTGGAACCGCTGCAGGAATCCCCGCGTCCGACCGOTGTTTGG
[0065] AAATCCCGTGAAGTTGCTAC3'
[0066] Pro4hum (SEQ ID NO5):
[0067] 5'CCCAAGCTTAACGCGGAGCACGCAGGGTGTACAGAACCATTTTACGGTGA
[0068] C...
Embodiment 2
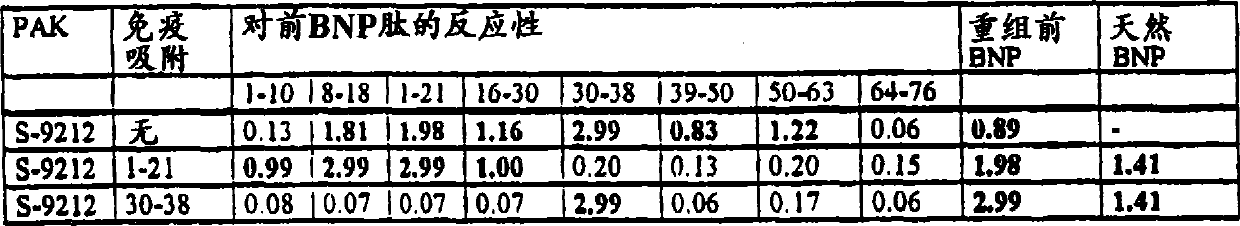
[0075] Production of polyclonal antibodies against N-terminal proBNP
[0076] 1. Immunization
[0077] Sheep were immunized with recombinant N-terminal proBNP(1-76) in complete Freund's adjuvant. The dose is 0.1 mg per animal. Immunizations were repeated at 4-week intervals over a 10-month period. Starting 6 weeks after the first immunization, serum was taken monthly and its sensitivity and titer determined.
[0078] 2. Purification of Polyclonal Antibody from Sheep Serum
[0079] Starting from the crude serum of sheep immunized with N-terminal proBNP, the lipid components were removed by a delipidation process performed in aerosol. Immunoglobulins were then isolated with ammonium sulfate (2M). 15 mM KPO at pH 7.0 4 , 50 mM NaCl and the dissolved precipitate was dialyzed and subjected to DEAE sepharose chromatography. In the IgG fraction, PAKS-IgG(DE) was present in the eluted fraction.
[0080] 3. Sequential affinity chromatography for the generation of NT-proBN...
Embodiment 3
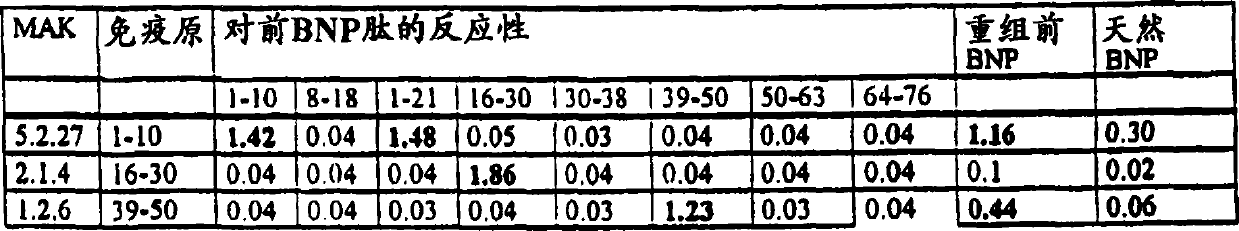
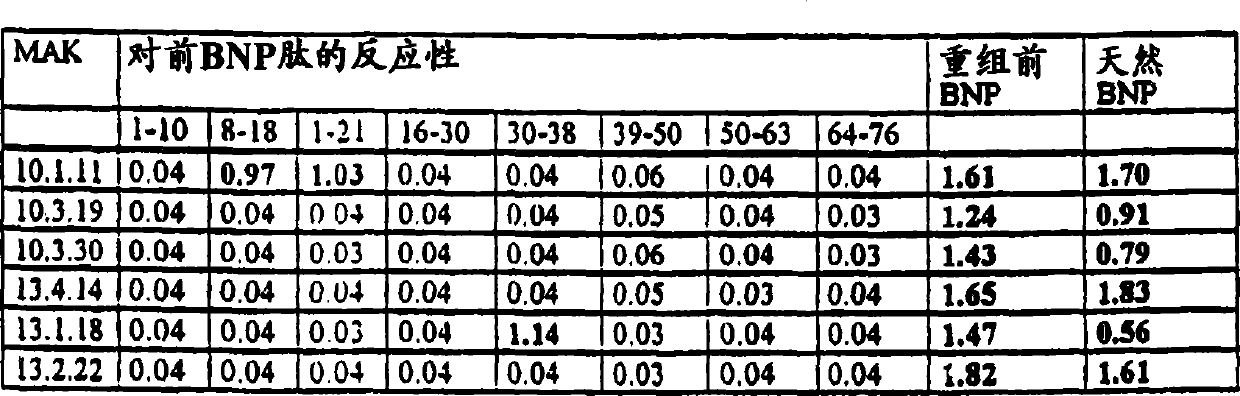
[0089] Production and detection of monoclonal antibody against N-terminal proBNP(1-76)
[0090] 1. Obtain monoclonal antibody against NT-proBNP(1-76)
[0091] 8-12 week old Balb / c mice were administered intraperitoneally with 100 μg of recombinant N-terminal proBNP antigen with Freund's adjuvant for immunization. After 6 weeks, 3 further immunizations were performed at 4 week intervals. Blood was drawn one week after the last immunization and the antibody titers in the sera of the test animals were determined. B-lymphocytes were obtained from the spleens of positive responding mice and fused with immortalized myeloma. The fusion is based on and the known method of Millstein (Nature 256, 1975, p.495-497). Primary cultures of hybrid cells constructed here are cloned by common means, for example, by use of commercially available cell sorters or by "limiting dilution". Only those clones, which react positively with recombinant N-terminal proBNP and recognize native N-termi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com