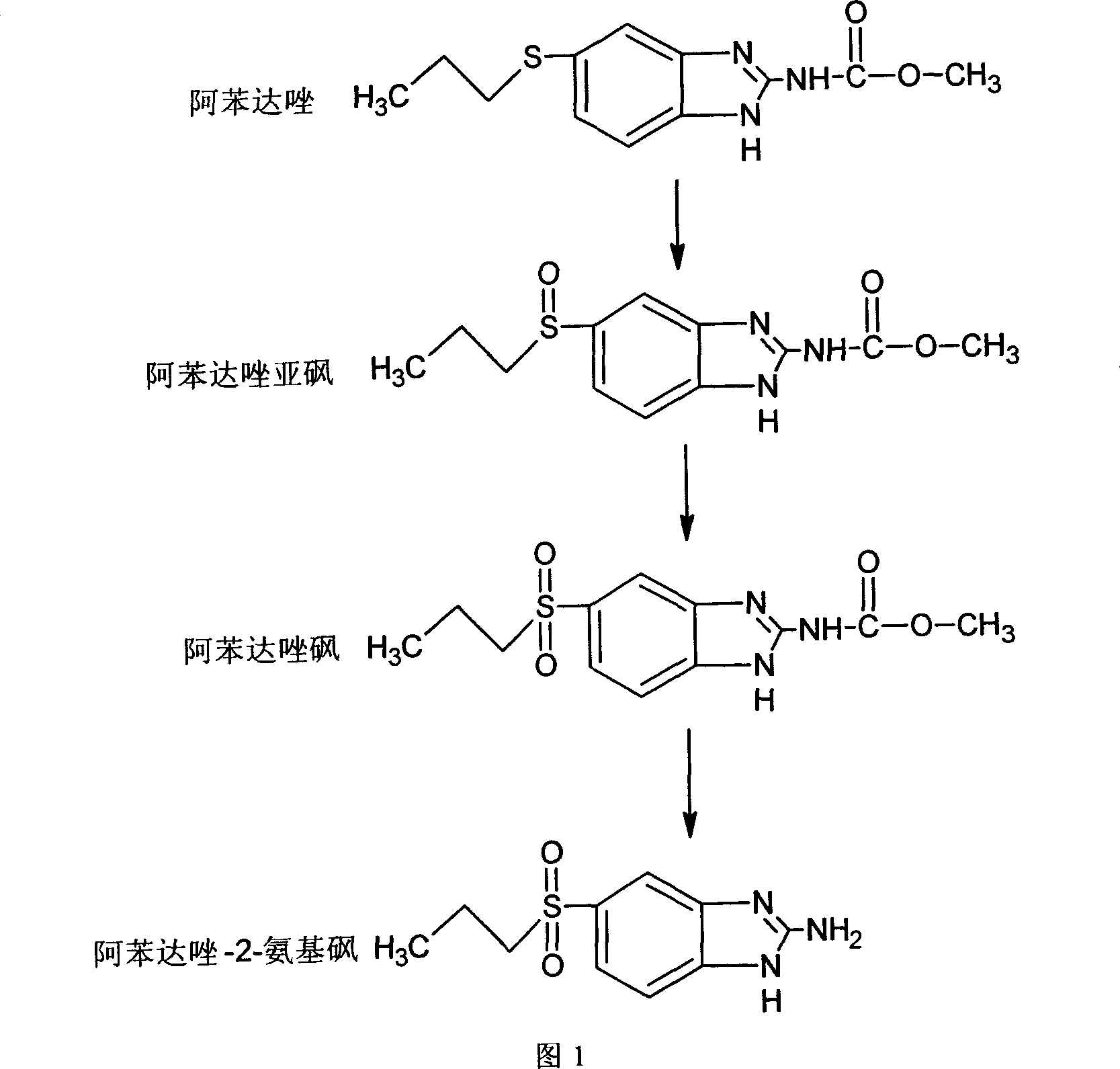
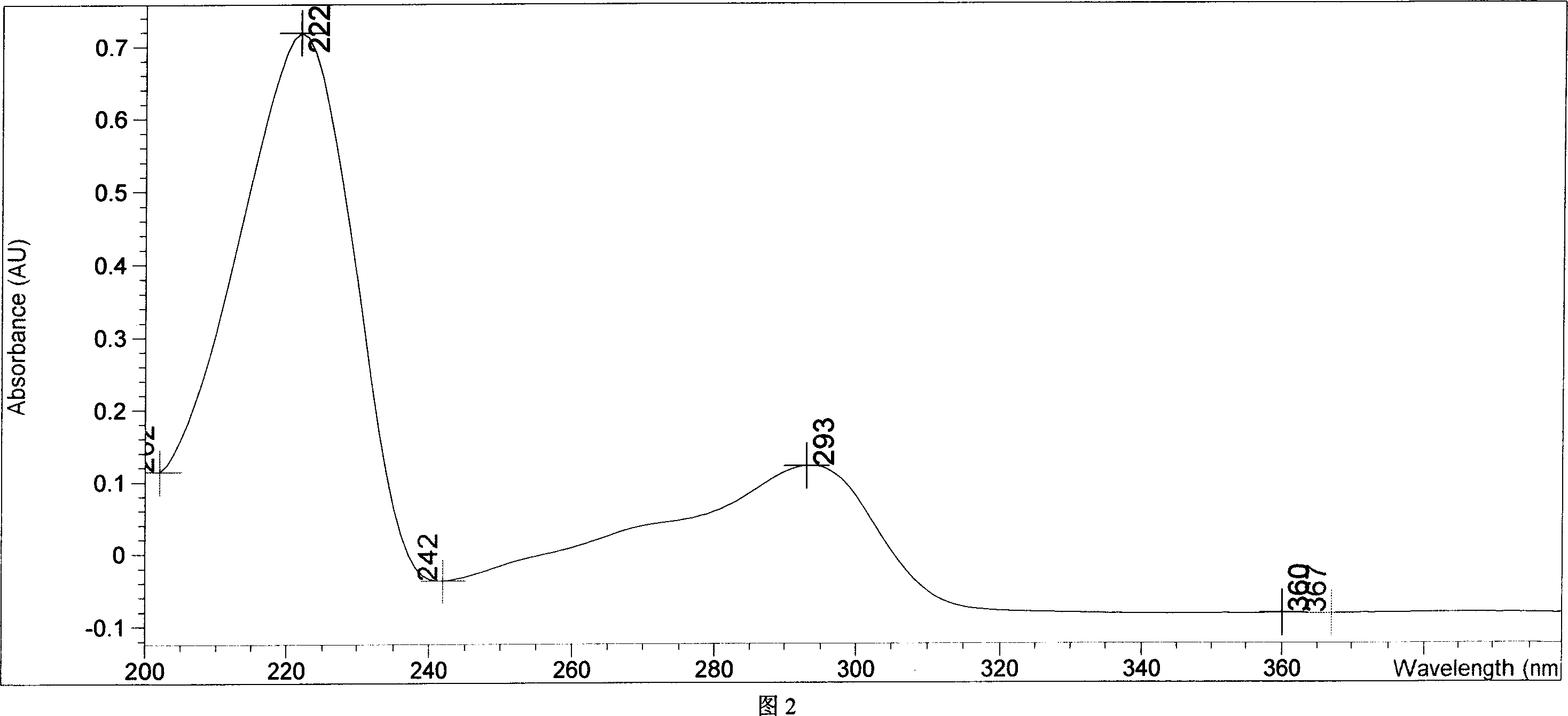
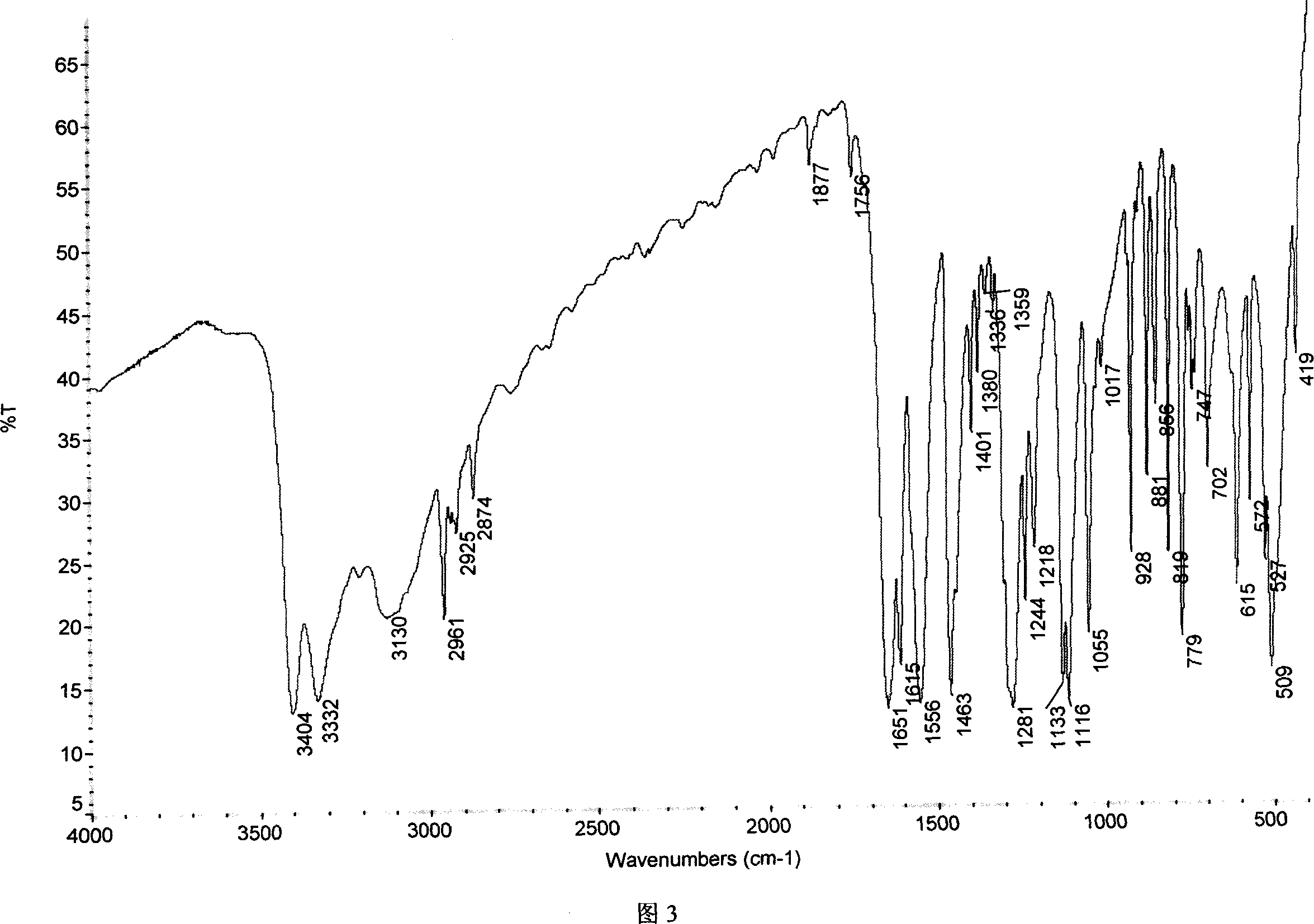
Chemical synthesis of albendazole-2-amino-sulphone
A technology of albendazole and chemical synthesis, applied in the field of chemical synthesis of albendazole-2-aminosulfone, one of the metabolites of albendazole
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 13.24g of albendazole into a 500ml four-neck flask equipped with a spherical condenser, then add 120mL of glacial acetic acid, and stir in a 60°C water bath until dissolved. First add 12mL of 30% hydrogen peroxide, then add 12mL of 30% hydrogen peroxide at an interval of 6 hours, then react for 6 hours, and react for a total of 12 hours to obtain a reaction mixture A. In 2mol·L -1 Neutralize the above reaction mixture A to pH 6.0 with a sodium hydroxide solution, filter, and dry the filter cake in an oven at 75-90°C. 12.45 g of crude albendazole sulfone was obtained.
[0029] Dissolve 10.03g of crude albendazole sulfone in 200mL0.68mol L -1 Add the hydrochloric acid solution into a 500mL four-necked flask equipped with a spherical condenser, stir and heat to reflux for 48h to obtain the reaction mixture B. -1 Neutralize the above reaction mixture B to pH 6.8 with sodium hydroxide solution, put it in a refrigerator at 4°C for 4 hours, filter, and dry the filter ca...
Embodiment 2
[0032] Add 13.26g of albendazole into a 500ml four-necked flask equipped with a spherical condenser, then add 120mL of glacial acetic acid, and stir in a water bath at 80°C until dissolved. First add 12mL of 30% hydrogen peroxide, then add 12mL of 30% hydrogen peroxide at an interval of 5h, then react for 5h, and react for a total of 10h to obtain a reaction mixture A. In 2mol·L -1 Neutralize the above reaction mixture A to pH 6.5 with a sodium hydroxide solution, filter, and dry the filter cake in an oven at 75-90°C to obtain 12.39 g of crude albendazole sulfone.
[0033] Dissolve 10.07g of crude albendazole sulfone in 150mL3.4mol L -1 Add the hydrochloric acid solution into a 500mL four-necked flask equipped with a spherical condenser, stir and heat to reflux for 36h to obtain the reaction mixture B, and use 2mol L -1 Neutralize the above reaction mixture B to pH 7.0 with sodium hydroxide solution, put it in a refrigerator at 4°C for 6 hours, filter, and dry the filter cak...
Embodiment 3
[0036] Add 13.25g of albendazole into a 500ml four-neck flask equipped with a spherical condenser, then add 120mL of glacial acetic acid in a 100°C water bath, and stir until dissolved. First add 12mL of 30% hydrogen peroxide, then add 12mL of 30% hydrogen peroxide at an interval of 4 hours, then react for 4 hours, and react for 8 hours to obtain the reaction mixture A. In 2mol·L -1 Neutralize the above reaction mixture A to pH 7.0 with a sodium hydroxide solution, filter, and dry the filter cake in an oven at 75-90°C. 12.18 g of crude albendazole sulfone was obtained.
[0037] Dissolve 10.01g of crude albendazole sulfone in 100mL6.8mol L -1 Add the hydrochloric acid solution into a 500mL four-necked flask equipped with a spherical condenser, stir and heat to reflux for 24h to obtain the reaction mixture B, and use 2mol L -1 Neutralize the above reaction mixture B to pH 7.8 with sodium hydroxide solution, put it in a refrigerator at 4°C for 12 hours, filter, and dry the fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com