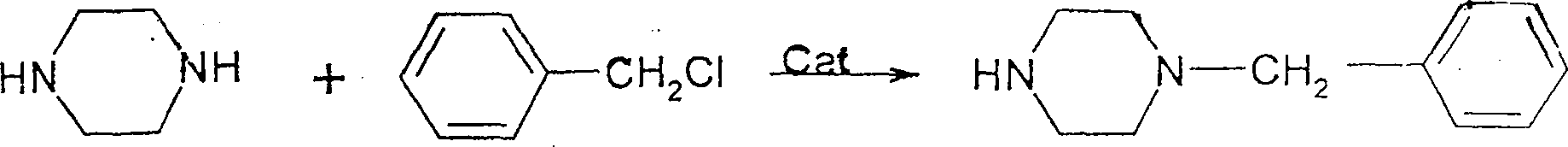Process for preparing N-benzyl piperazine
A technology of benzylpiperazine and piperazine, which is applied in the field of preparation of N-benzylpiperazine, can solve the problems of low yield, complicated operation and low product purity, and achieves a high yield, simple operation and high purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0019] In a 500ml reaction bottle, drop 25.8g (0.3mol) of piperazine and 100ml of solvent anhydrous methanol, stir and dissolve, then drop into the catalyst aniline hydrochloride, heat up to 50 degrees, drop 38g (0.3mol) of benzyl chloride, about Add dropwise in half an hour. Keep the temperature at 50°C for 3 hours, cool to normal temperature, reduce the pressure to 15mmhg, and evaporate to remove the solvent methanol. Then add NaOH solution, adjust the pH value to 13, and extract twice with ethyl acetate, each with 50 ml of ethyl acetate. Distill the extract under normal pressure, reduce the pressure to 2.5 mmhg, and collect fractions at 120—124°C by distillation to finally obtain 50.5 g of the target product, N-benzylpiperazine, with a yield of 95.5% and a purity of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com