Super-phthalocyanine compound with six isoindole structure subunits in laver oxazine cycle and its synthesis and use
A compound, pyrazine dicarboxylic acid technology, applied in cobalt organic compounds, nickel organic compounds, copper organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Compound 1a 1 Synthesis
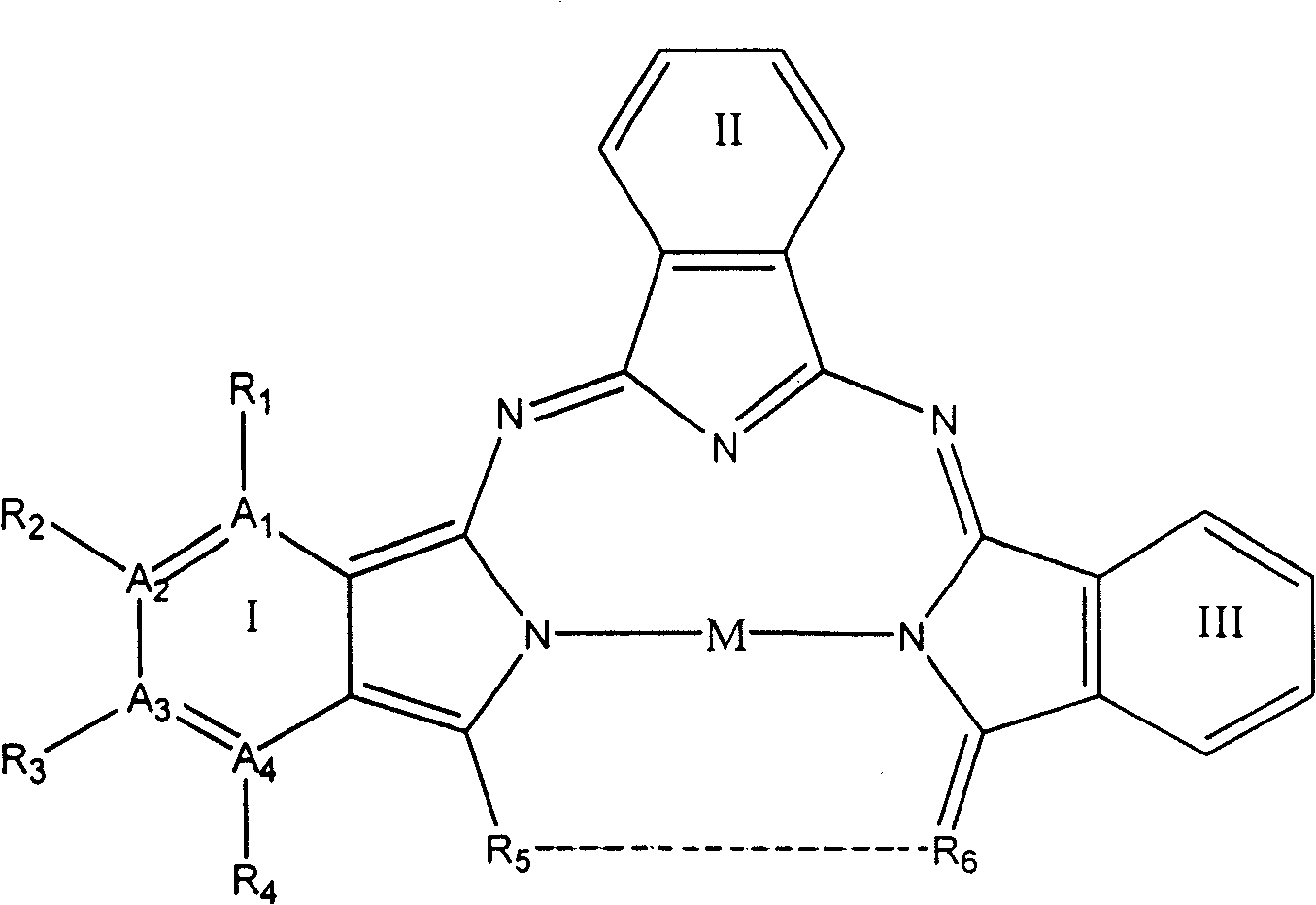
[0053] Select A in general formula (1) 1 and A 4 stands for nitrogen atom, A 2 and A 3 stands for carbon atom, R 1 and R 4 does not represent any group or atom, R 2 and R 3 represents hydrogen atom, M represents Cu(II) ion, R 5 …R 6 represent
[0054]
[0055] The structures of parts I, II, and III are identical and constitute compound 1a 1 , the structure is shown in Figure 4.
[0056] Weigh 8.0001g of urea, compound of general formula (3) such as: 2,3-pyrazine-2,3-dicarboxylic Acid 4.0008g (0.0238mol), CuCl 2 ·2H 2 O1.3683g (0.0080mol), (NH 4 ) 2 MoO 4 0.8001g was finely ground and mixed evenly, put it into a steel bomb with a teflon lining and sealed, put the steel bomb in an oven and heated to 240°C for 8 hours; open the lid after the steel bomb cools to room temperature, and take out the black solid; The black solid was placed in a vacuum purification unit, and at 10 -4 Pa, purified at 300°C for 24 hours. Co...
Embodiment 2
[0057] Example 2 Compound 2a 1 Synthesis
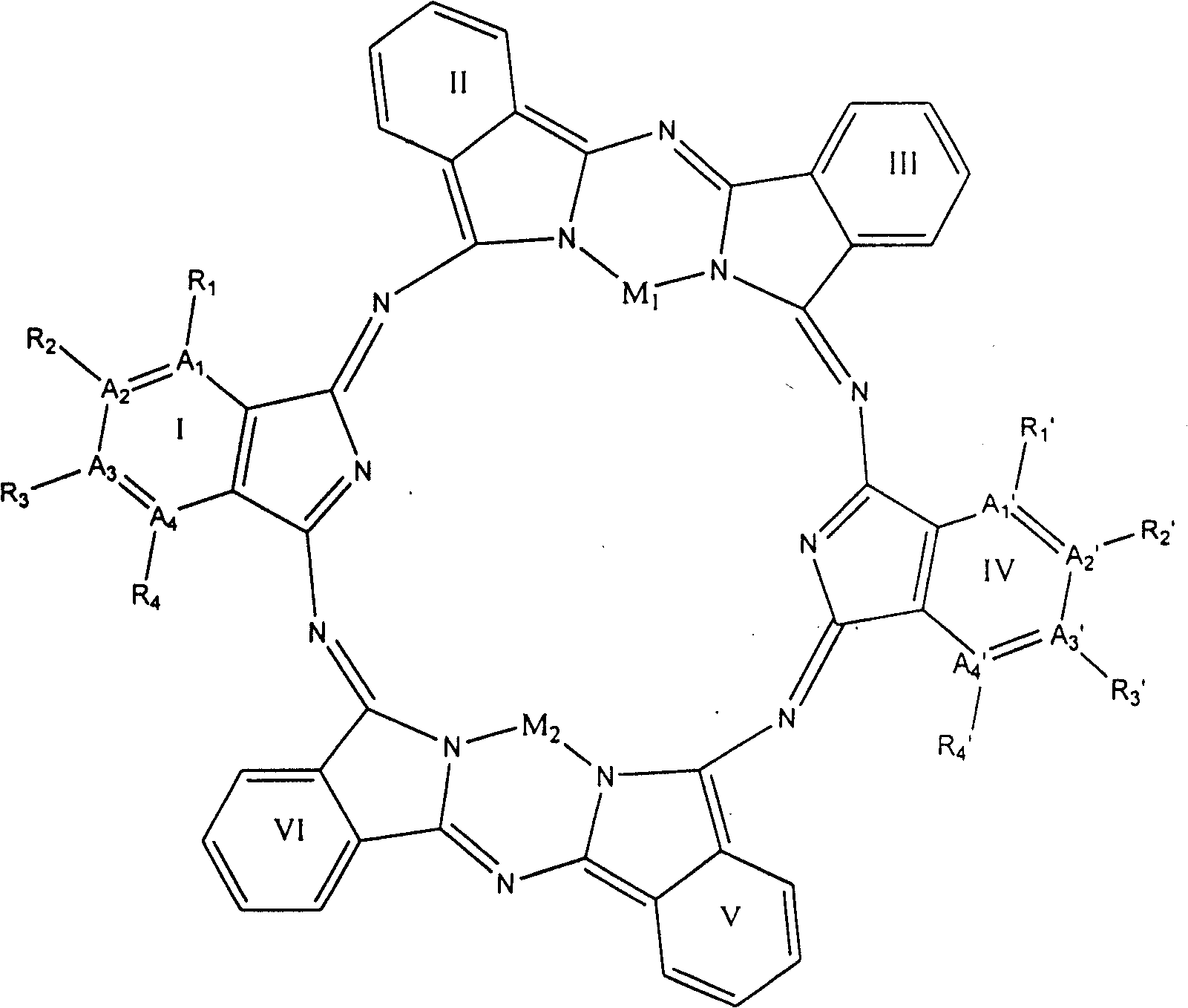
[0058] Select A in general formula (1) 1 and A 4 stands for nitrogen atom, A 2 and A 3 stands for carbon atom, R 1 and R 4 does not represent any group or atom, R 2 and R 3 Represents a hydrogen atom, M represents a Cu(II) ion, I, II, III, IV, V, VI have the same structure, forming the compound 2a of the general formula (2) 1 , the structure is shown in Figure 8
[0059] The prepared compound 1a of general formula (1) 1 into the vacuum, at 10 -4 Pa, reacted at 500°C for 24 hours to generate compound 1a of general formula (1) 1 After the bimolecular condensation reaction of , the compound 2a of the general formula (2) was prepared 1 . The compound 2a of the general formula (2) was measured by mass spectrometer 1 The molecular ion peak of (Fig. 9) M+Na + : 931.9193amu, close to the calculated value of 930.7240, and M+H+Na + The calculated value of 931.7319 matches. This is attributed to hydrogen gas in the mass spectrom...
Embodiment 3
[0060] Embodiment 3 General formula (2) compound 2a 1 UV-VIS-NIR absorption spectrum
[0061] Study on compound 2a of general formula (2) by UV-VIS-NIR spectrometer 1 UV-VIS-NIR absorption spectrum of formic acid solution ( Figure 16 ), and the results show that in addition to the absorption peaks in the ultraviolet region and visible region shared by general phthalocyanines, there is an absorption in the near-red region (1.428μm), and the absorption coefficient is very large. Compound 2a of general formula (2) we observed 1 The UV-VIS-NIR absorption spectrum of phthalocyanine is very different from that of general phthalocyanine. Using this property, the compound of general formula (2) can be used to prepare optoelectronic devices, especially near-infrared optoelectronic devices. Compound 2a of general formula (2) 1 When the photoactivity in the near-infrared region is used for the infrared photodynamic therapy of cancer, the light transmission process can be completed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com