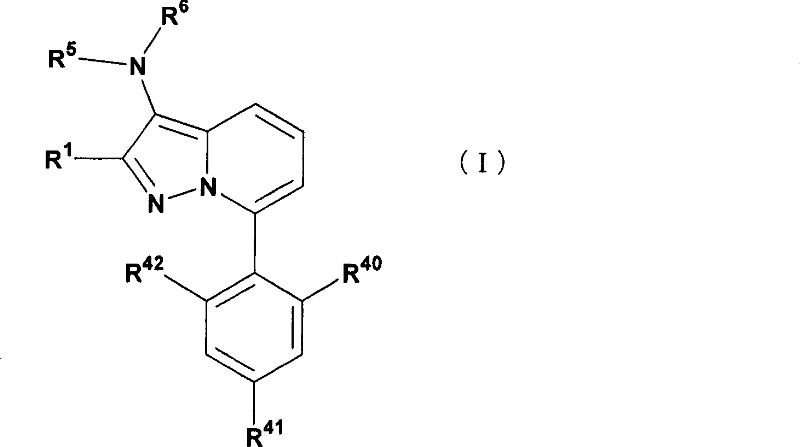
7-phenylpyrazolopyridine compounds
A compound, methyl technology, applied in the field of 7-phenylpyrazolopyridine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0236] The following Preparation Examples, Examples and Test Examples are used for illustration only and do not impose any limitation on the compounds of the present invention. It is obvious to those skilled in the art that various modifications can be made to these examples within the scope of the claims of the present invention to maximize the effect of the present invention, and these modifications are also included in the scope of the claims.
[0237] The idiom "purify by silica gel column chromatography and obtain the title compound from fractions" in this specification means that the fraction containing the target compound obtained by silica gel column chromatography is concentrated and further recrystallized if necessary Purification affords the title compound.
preparation Embodiment 1
[0239] 2-(1-butynyl)pyridine
[0240]
[0241] Add dichlorobis(triphenylphosphine)palladium(II) (2.2g) and cuprous iodide (0.3g) in the solution that 2-bromopyridine (50g) is dissolved in diethylamine (500mL), reaction mixture is in Stir at room temperature for 4 hours while introducing gaseous 1-butyne (100 g). The resulting reaction mixture was bubbled with nitrogen, then ethyl acetate was added. The reaction mixture was filtered through celite to remove insoluble residue, and the filtrate was washed with water and brine. The organic extract was dried over anhydrous magnesium sulfate and filtered, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography to obtain the title compound (35 g) as a brown oil from n-hexane:ethyl acetate (5:1) fractions .
[0242] 1 H NMR (400MHz, CDCl 3 )δ1.26(t, J=7.6Hz, 3H), 2.45(q, J=7.6Hz, 2H), 7.16-7.20(m, 1H), 7.35-7.38(m, 1H), 7.59-7.63(m , 1H), 8.53-8.54(m, 1H).
preparation Embodiment 2
[0244] 2-Ethylpyrazolo[1,5-a]pyridine
[0245]
[0246] To a solution of 2-(1-butynyl)pyridine (12.8 g) dissolved in dichloromethane (60 mL) was added O-mesitylenesulfonyl hydroxylamine (reference: Synthesis, 1997, 1) (20 g) solution in dichloromethane (132 mL) while cooling with ice, and the reaction mixture was stirred for 30 minutes. Diethyl ether (2 L) was added to the reaction mixture to precipitate crystals, which were filtered and dried under reduced pressure to give crude N-amino-2-(1-butynyl)pyridinium mesitylenesulfonate (12.6 g) as a colorless crystals.
[0247] 6.1 g of crude N-amino-2-(1-butynyl)pyridinium mesitylenesulfonate was dissolved in tetrahydrofuran (600 mL), potassium tert-butoxide (3.55 g) was added at room temperature, and the reaction mixture was vigorously stirred for 30 minutes . Then ice water was added and extracted with ethyl acetate. After extracting the aqueous phase twice with ethyl acetate, the insoluble residue was filtered with diato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com