Battery acid, commonly referring to sulfuric acid (H₂SO₄) used in lead-acid batteries, is a fundamental component in electrochemical power systems. As energy storage demands expand across automotive, renewable, and backup power markets, understanding battery acid’s function becomes essential.
This article explores the chemistry, performance attributes, industrial uses, comparative advantages, and future innovations surrounding battery acid—guided by PatSnap’s Eureka AI Agent.
Composition & Core Properties
Battery acid is primarily composed of diluted sulfuric acid, typically around 30–38% H₂SO₄ by weight. Its role is to enable ionic conduction between the lead-based electrodes inside the battery during charge and discharge cycles.
Key Properties:
- Strong electrolyte: Enables efficient ion transfer
- Highly corrosive: Reacts with metals and organic material
- Thermal sensitivity: Generates heat under load or charging
- Density-dependent state-of-charge indicator: Electrolyte density correlates with battery charge level
Comparative Advantages & Limitations
Advantages
- Proven Reliability Across Applications
Battery acid (diluted sulfuric acid) has powered lead-acid systems for over a century, demonstrating consistent performance in automotive, industrial, and grid applications under various environmental conditions. - Low Cost and Readily Available Materials
Sulfuric acid is inexpensive to produce, and the global infrastructure for lead-acid battery manufacture and recycling is mature—leading to one of the lowest costs per kilowatt-hour (kWh) in energy storage. - High Discharge Capability
Lead-acid batteries using sulfuric acid deliver high peak currents, making them ideal for cranking engines or providing surge power in backup systems. - Ease of Recyclability
Over 95% of lead-acid batteries (including the acid) are recycled worldwide. Sulfuric acid can be neutralized or reused, supporting closed-loop sustainability models. - Chemically Stable at Room Temperature
Compared to lithium-ion electrolytes, sulfuric acid is non-flammable and thermally stable under normal operating conditions, reducing fire risks.

Limitations
- Corrosive Nature and Health Hazards
Sulfuric acid is highly corrosive and poses significant risks upon skin contact, inhalation, or eye exposure. Hydrogen gas evolution during charging introduces explosion risks if ventilation is poor. - Lower Energy and Power Density
Compared to modern lithium-ion batteries, sulfuric acid systems offer inferior energy density (~30–40 Wh/kg), making them unsuitable for weight- or volume-constrained applications like mobile electronics or aviation. - Degradation Mechanisms
Over time, issues like acid stratification, sulfation of plates, and water loss degrade battery performance. These mechanisms reduce charge acceptance and cycle life, especially under deep discharge conditions. - Environmental and Transport Regulations
Battery acid is classified as a hazardous substance, requiring strict adherence to regulations (e.g., DOT, EPA, ADR). Special handling, labeling, and neutralization protocols are necessary in transport, storage, and disposal. - Temperature Sensitivity and Performance Loss
Electrolyte viscosity and conductivity drop significantly in cold environments, impairing charge/discharge rates. Conversely, elevated temperatures accelerate corrosion and electrolyte evaporation. - Limited Deep-Cycle Durability
Standard flooded lead-acid batteries using sulfuric acid typically offer ~300–500 cycles at 50% depth of discharge (DoD), which is lower than advanced chemistries like LiFePO₄ or nickel-metal hydride.
Application Domains
Battery acid, primarily composed of sulfuric acid, is a critical component in electrochemical systems across diverse industries. Its ability to facilitate ion transport and influence cell chemistry makes it indispensable in energy, electronics, environmental, and automotive applications. Below are key sectors where battery acid plays a transformative role:
Energy Storage & Conversion
The rising global demand for renewable energy calls for materials that can store and convert energy efficiently. Battery acid is fundamental in enabling redox reactions in electrochemical systems, directly impacting energy density, cycle life, and efficiency.
In current research, efforts are concentrated on refining acid composition for advanced battery systems such as lithium-ion and vanadium redox flow batteries. Priorities include enhancing thermal stability, minimizing degradation, and increasing recyclability.
Research Frontlines
Ongoing work targets:
- Optimizing acid formulations for high-efficiency energy storage
- Developing non-corrosive and encapsulated alternatives
- Improving lifecycle performance of flow and sealed batteries
Related Reports
- Advances in Battery Acid Recycling Technologies
- The Role of Battery Acid in Lithium-Ion Cells
- Battery Acid Decomposition: Kinetics and Mechanisms
- Innovations in Battery Acid Encapsulation Technologies
- Battery Acid in High-Temperature Electrolytes: Analysis and Efficiency
- Impact of Battery Acid Concentration on Cell Voltage Output
- Battery Acid Synthesis Routes and Their Efficiency Impacts
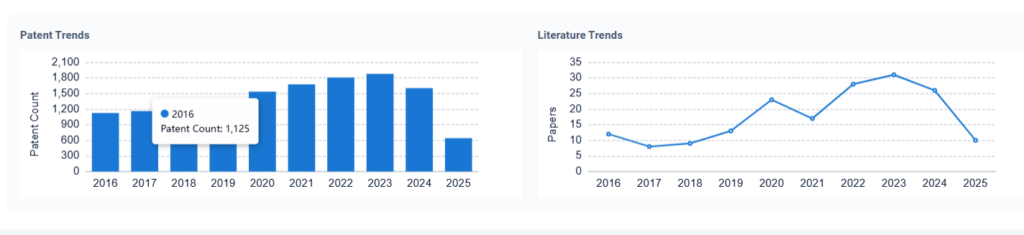
Environmental Impact & Safety
As environmental regulations tighten, managing the life cycle of battery acid has become a pressing priority. Improper disposal can lead to soil acidification, aquatic toxicity, and long-term ecosystem disruption.
Innovations focus on leak-proof containment, neutralization technologies, and safe transportation protocols. The development of absorbent materials and early-warning spill detection systems are also gaining traction.
Research Frontlines
Key directions include:
- Spill mitigation strategies
- Marine impact assessments
- Safety standards for acid waste logistics
Related Reports
- Battery Acid Containment Solutions for Environmental Safety
- Standards for Battery Acid Disposal in Marine Environments
- Advances in Battery Acid Spill Containment Materials
- Strategies to Facilitate Battery Acid Recovery in Remote Areas
- How to Safely Transport Battery Acid in Bulk
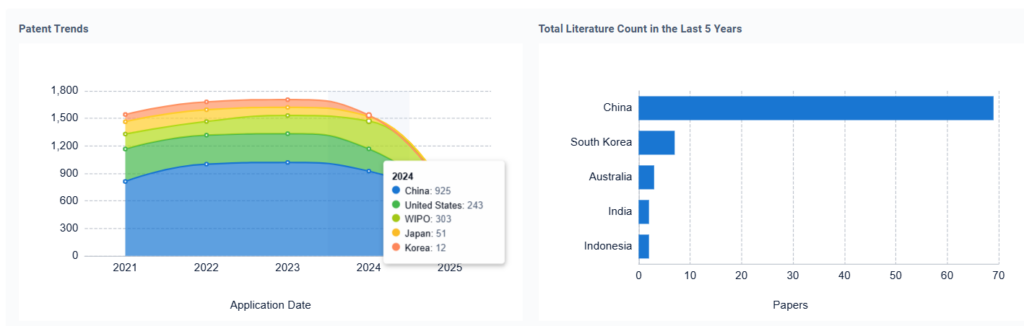
Advanced Electronics
Battery acid significantly influences electrolyte behavior in next-generation electronics. With growing demand for compact and reliable devices, understanding how sulfuric acid interacts with conductive polymers, nanomaterials, and semiconductors is essential.
The main challenge lies in mitigating acid corrosion while maximizing conductivity. Materials research is geared toward designing acid-tolerant composites and improving ionic pathways in flexible and wearable electronics.
Research Frontlines
Promising avenues include:
- Enhancing acid-electrolyte stability
- Acid-resistant membrane development
- Nanomaterial-integrated electrolyte systems
Related Reports:
- How Battery Acid Influences Polymer Electrolyte Membranes
- How Nanomaterials Enhance Battery Acid Effectiveness
- Battery Acid’s Role in Conductive Additive Performance
- Effects of Battery Acid on Semiconductor Derivatives
Automotive & Transportation
Battery acid remains at the core of lead-acid and emerging battery chemistries used in electric and hybrid vehicles. Its role in powering starter motors, managing charge cycles, and supporting regenerative braking systems is critical to modern mobility.
Current trends involve improving thermal resistance, minimizing acid stratification, and enhancing charge retention. As the EV market grows, research focuses on acid stability under rapid cycling and across a wide temperature range.
Research Frontlines
Industry focus areas:
- Lifecycle extension in mobility batteries
- Acid composition and its impact on EV charge dynamics
- Integration into fuel cell and hybrid systems
Related Reports:
- Battery Acid’s Role in Hybrid Vehicle Energy Systems
- Impact of Battery Acid Concentration on Cell Voltage Output
- How Battery Acid Supports Power Grid Stabilization
- Comparative Study of Battery Acid Reactivity at High Temperatures
- Role of Battery Acid in Fuel Cell Technologies
- How Battery Acid Affects Supercapacitor Performance
Cross-Sector Innovation
Battery acid is not limited to traditional power systems. Its electrochemical versatility holds untapped potential across telecom, aerospace, maritime, and off-grid energy applications—but where exactly are the next breakthroughs?
That’s where PatSnap Eureka AI Agent comes in.
With Eureka, you can:
- Discover cross-sector use cases hidden across global patents, scientific papers, and industry disclosures
- Identify white space opportunities in acid formulation, containment, and multi-environment deployment
- Benchmark how leading innovators are repurposing sulfuric acid chemistries for next-gen applications
👉 Want to uncover what others can’t see?
Use PatSnap’s Eureka AI Agent to chart your own roadmap for sulfuric acid innovation—beyond the obvious.
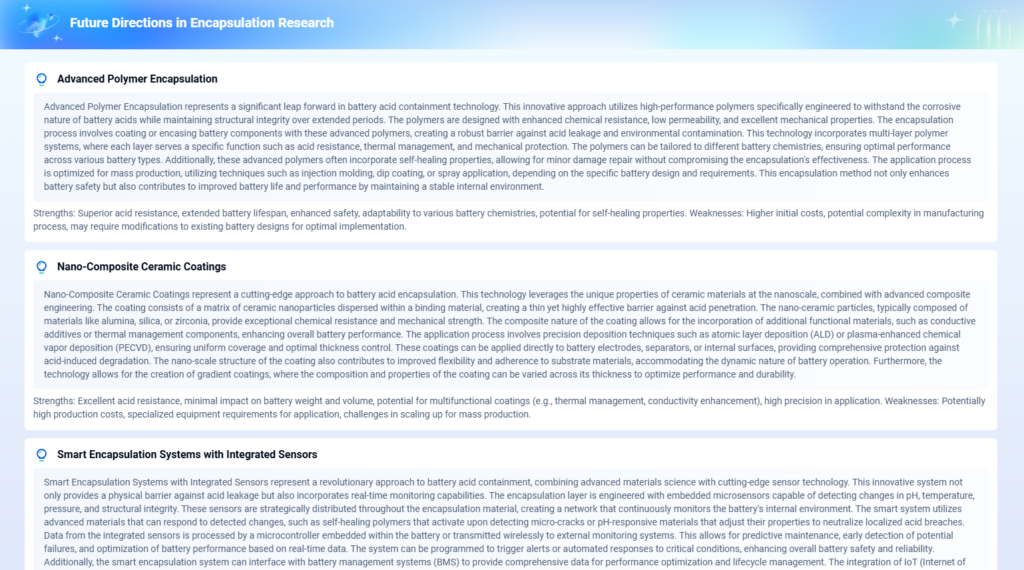
Future Outlook & Research Frontiers
As pressure grows for safer, more sustainable battery technologies, sulfuric acid-based systems are evolving in two directions:
- Advanced AGM & Gel Batteries: Use modified battery acid formulations to improve safety, reduce leakage, and enhance cycle life.
- Electrolyte Optimization: Innovations in additives (e.g., phosphoric acid blends or silica gels) improve thermal management and reduce sulfation.
Emerging Trends:
- Lead-carbon hybrids to reduce acid stratification
- Closed-loop recycling of battery acid
- Safer transportation and containment systems
- Non-toxic acid analogs under early-stage research
Conclusion & Strategic Takeaways
Battery acid remains an essential material for global energy storage infrastructure. While newer chemistries like lithium-ion dominate innovation headlines, sulfuric acid-powered systems offer unmatched affordability, recyclability, and robustness in legacy and emerging contexts alike.
From EV backup batteries to rural solar grids, understanding and optimizing battery acid is critical to sustainable electrification strategies.
FAQ: Battery Acid Insights
A: Usually 30–38% sulfuric acid by weight in water.
A: Yes, both the lead and acid can be recovered and reused in closed-loop systems.
A: Highly corrosive. It can cause severe burns and emits hydrogen gas during operation, requiring PPE and ventilation.
A: No. Lithium-ion batteries use non-aqueous organic electrolytes, not sulfuric acid.
A: It can corrode metals and concrete, and poses a chemical burn hazard. Spill kits and neutralization agents are essential.
A: Yes. SLA and gel types minimize acid spillage and gas emissions, making them safer for enclosed environments.
A: Yes. Transport, storage, and disposal are subject to regulations like OSHA, DOT, and REACH due to its corrosivity and environmental impact.
Battery acid technologies continue to evolve rapidly across chemistry, safety, and performance fronts. With PatSnap Eureka AI Agent , you can:
- Map real-time R&D and patent activity in battery electrolytes
- Benchmark key players driving sulfuric acid innovations
- Identify trends in lead-acid lifecycle extension and safe handling
👉 Explore next-gen battery acid applications now.