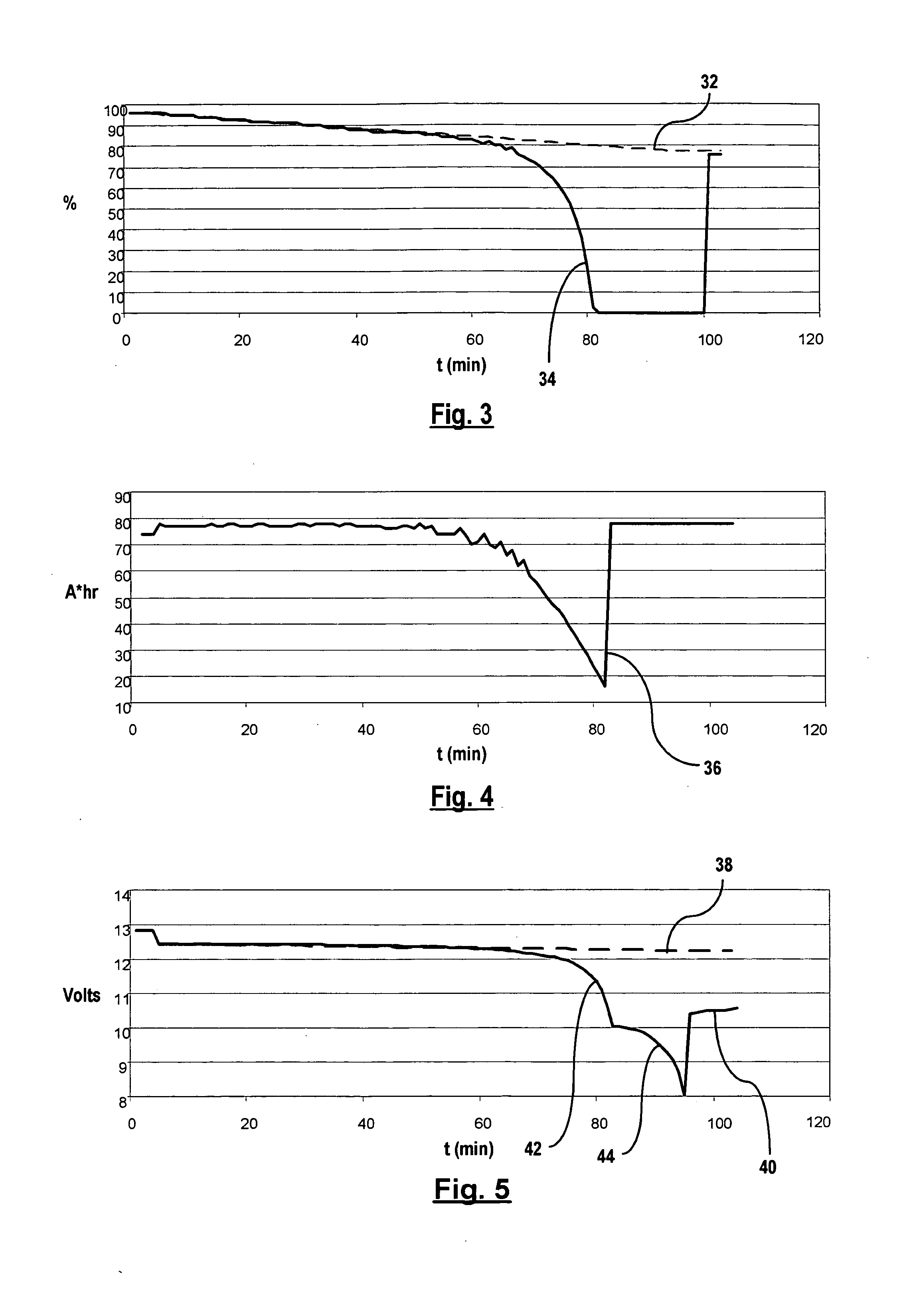
Impact of Battery Acid Concentration on Cell Voltage Output
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Battery Acid Evolution
The evolution of battery acid concentration has played a crucial role in the development and optimization of battery technology. Initially, lead-acid batteries used a simple mixture of sulfuric acid and water as the electrolyte. However, as research progressed, scientists discovered that the concentration of acid significantly impacted cell voltage output and overall battery performance.
In the early stages of battery development, researchers experimented with various acid concentrations to find the optimal balance between power output and battery longevity. They observed that higher acid concentrations generally led to increased voltage output and improved battery capacity. This discovery prompted a shift towards using more concentrated acid solutions in battery manufacturing.
As the understanding of electrochemistry advanced, scientists began to explore the relationship between acid concentration and the chemical reactions occurring within the battery. They found that the concentration of sulfuric acid directly affected the rate of the redox reactions at the electrodes, influencing both the battery's power output and its ability to hold a charge over time.
The advent of more sophisticated analytical techniques in the mid-20th century allowed researchers to study the effects of acid concentration on a molecular level. This led to a deeper understanding of how acid concentration impacts the formation and dissolution of lead sulfate crystals on the battery plates, a critical factor in determining battery life and performance.
In recent decades, the focus has shifted towards optimizing acid concentration for specific battery applications. For instance, deep-cycle batteries used in renewable energy systems require a different acid concentration compared to starter batteries used in automobiles. This tailored approach has resulted in the development of batteries with improved efficiency and longer lifespans for various use cases.
Advancements in materials science have also contributed to the evolution of battery acid technology. The introduction of gel electrolytes and absorbed glass mat (AGM) designs has allowed for the use of higher acid concentrations without the risk of acid spillage or leakage. These innovations have not only improved battery performance but also enhanced safety and reliability.
Today, researchers continue to explore novel electrolyte compositions and concentrations to push the boundaries of battery technology. The ongoing quest for higher energy density, faster charging times, and longer cycle life drives the constant evolution of battery acid formulations. As we move towards more sustainable energy solutions, the optimization of battery acid concentration remains a critical area of research in the pursuit of more efficient and powerful energy storage systems.
In the early stages of battery development, researchers experimented with various acid concentrations to find the optimal balance between power output and battery longevity. They observed that higher acid concentrations generally led to increased voltage output and improved battery capacity. This discovery prompted a shift towards using more concentrated acid solutions in battery manufacturing.
As the understanding of electrochemistry advanced, scientists began to explore the relationship between acid concentration and the chemical reactions occurring within the battery. They found that the concentration of sulfuric acid directly affected the rate of the redox reactions at the electrodes, influencing both the battery's power output and its ability to hold a charge over time.
The advent of more sophisticated analytical techniques in the mid-20th century allowed researchers to study the effects of acid concentration on a molecular level. This led to a deeper understanding of how acid concentration impacts the formation and dissolution of lead sulfate crystals on the battery plates, a critical factor in determining battery life and performance.
In recent decades, the focus has shifted towards optimizing acid concentration for specific battery applications. For instance, deep-cycle batteries used in renewable energy systems require a different acid concentration compared to starter batteries used in automobiles. This tailored approach has resulted in the development of batteries with improved efficiency and longer lifespans for various use cases.
Advancements in materials science have also contributed to the evolution of battery acid technology. The introduction of gel electrolytes and absorbed glass mat (AGM) designs has allowed for the use of higher acid concentrations without the risk of acid spillage or leakage. These innovations have not only improved battery performance but also enhanced safety and reliability.
Today, researchers continue to explore novel electrolyte compositions and concentrations to push the boundaries of battery technology. The ongoing quest for higher energy density, faster charging times, and longer cycle life drives the constant evolution of battery acid formulations. As we move towards more sustainable energy solutions, the optimization of battery acid concentration remains a critical area of research in the pursuit of more efficient and powerful energy storage systems.
Market Demand Analysis
The market demand for batteries with optimized acid concentration and voltage output is experiencing significant growth across various sectors. This surge is primarily driven by the increasing adoption of electric vehicles (EVs), renewable energy storage systems, and portable electronic devices. As these industries continue to expand, the need for high-performance, long-lasting batteries becomes more critical.
In the automotive sector, the global EV market is projected to grow at a compound annual growth rate (CAGR) of over 20% in the coming years. This rapid expansion is creating a substantial demand for batteries with improved voltage output and efficiency. Manufacturers are actively seeking solutions to enhance battery performance, with acid concentration optimization being a key area of focus.
The renewable energy sector is another major driver of market demand for advanced battery technologies. As countries worldwide transition towards cleaner energy sources, the need for efficient energy storage solutions is escalating. Grid-scale battery storage systems, which rely heavily on optimized battery performance, are becoming increasingly prevalent. The market for these systems is expected to grow significantly, further fueling the demand for batteries with improved acid concentration and voltage output characteristics.
In the consumer electronics market, the demand for longer-lasting and faster-charging batteries continues to rise. Smartphones, laptops, and other portable devices are constantly evolving, requiring more power-efficient batteries. This trend is pushing manufacturers to explore various methods of enhancing battery performance, including optimizing acid concentration to improve voltage output and overall efficiency.
The industrial sector is also contributing to the growing demand for advanced battery technologies. Applications such as uninterruptible power supplies (UPS), material handling equipment, and backup power systems require batteries with consistent and reliable voltage output. The optimization of acid concentration plays a crucial role in meeting these requirements and improving overall system performance.
Furthermore, the aerospace and defense industries are showing increased interest in high-performance batteries. These sectors demand batteries with exceptional reliability, longevity, and power output for various applications, ranging from satellites to military equipment. The optimization of battery acid concentration to enhance voltage output is a key area of research and development in these fields.
As environmental concerns continue to grow, there is an increasing emphasis on developing more sustainable and efficient battery technologies. This trend is driving research into optimizing battery components, including acid concentration, to improve overall performance and reduce environmental impact. The market is showing a clear preference for batteries that offer higher energy density, longer lifespan, and improved safety profiles.
In conclusion, the market demand for batteries with optimized acid concentration and improved voltage output is robust and diverse. From electric vehicles to renewable energy storage, and from consumer electronics to industrial applications, the need for advanced battery technologies is evident across multiple sectors. This demand is expected to continue growing, driving further innovation and research in battery optimization techniques.
In the automotive sector, the global EV market is projected to grow at a compound annual growth rate (CAGR) of over 20% in the coming years. This rapid expansion is creating a substantial demand for batteries with improved voltage output and efficiency. Manufacturers are actively seeking solutions to enhance battery performance, with acid concentration optimization being a key area of focus.
The renewable energy sector is another major driver of market demand for advanced battery technologies. As countries worldwide transition towards cleaner energy sources, the need for efficient energy storage solutions is escalating. Grid-scale battery storage systems, which rely heavily on optimized battery performance, are becoming increasingly prevalent. The market for these systems is expected to grow significantly, further fueling the demand for batteries with improved acid concentration and voltage output characteristics.
In the consumer electronics market, the demand for longer-lasting and faster-charging batteries continues to rise. Smartphones, laptops, and other portable devices are constantly evolving, requiring more power-efficient batteries. This trend is pushing manufacturers to explore various methods of enhancing battery performance, including optimizing acid concentration to improve voltage output and overall efficiency.
The industrial sector is also contributing to the growing demand for advanced battery technologies. Applications such as uninterruptible power supplies (UPS), material handling equipment, and backup power systems require batteries with consistent and reliable voltage output. The optimization of acid concentration plays a crucial role in meeting these requirements and improving overall system performance.
Furthermore, the aerospace and defense industries are showing increased interest in high-performance batteries. These sectors demand batteries with exceptional reliability, longevity, and power output for various applications, ranging from satellites to military equipment. The optimization of battery acid concentration to enhance voltage output is a key area of research and development in these fields.
As environmental concerns continue to grow, there is an increasing emphasis on developing more sustainable and efficient battery technologies. This trend is driving research into optimizing battery components, including acid concentration, to improve overall performance and reduce environmental impact. The market is showing a clear preference for batteries that offer higher energy density, longer lifespan, and improved safety profiles.
In conclusion, the market demand for batteries with optimized acid concentration and improved voltage output is robust and diverse. From electric vehicles to renewable energy storage, and from consumer electronics to industrial applications, the need for advanced battery technologies is evident across multiple sectors. This demand is expected to continue growing, driving further innovation and research in battery optimization techniques.
Current Challenges
The current challenges in the field of battery acid concentration and its impact on cell voltage output are multifaceted and complex. One of the primary issues is the difficulty in maintaining optimal acid concentration levels over the battery's lifecycle. As batteries discharge and recharge, the acid concentration can fluctuate, leading to inconsistent voltage output and reduced overall performance.
Another significant challenge is the corrosive nature of battery acid, which can lead to degradation of battery components over time. This corrosion can affect the electrodes, separators, and other internal structures, ultimately impacting the battery's ability to maintain a stable voltage output. Researchers and manufacturers are continuously seeking ways to mitigate this corrosion while maintaining the necessary acid concentration for optimal performance.
The environmental impact of battery acid is also a pressing concern. Improper disposal or leakage of battery acid can have severe consequences for ecosystems and human health. This challenge extends beyond the technical aspects of battery performance and into the realm of environmental regulations and sustainable manufacturing practices.
Temperature sensitivity presents another hurdle in managing battery acid concentration. Extreme temperatures can affect the acid's concentration and reactivity, leading to variations in voltage output. This is particularly problematic in applications where batteries are exposed to wide temperature ranges, such as in automotive or outdoor energy storage systems.
The pursuit of higher energy densities in batteries also complicates the management of acid concentration. As manufacturers strive to pack more power into smaller battery packages, the balance between acid concentration and other battery components becomes increasingly delicate. This challenge requires innovative approaches to electrolyte design and cell architecture.
Standardization of measurement and control methods for acid concentration across different battery types and applications remains an ongoing challenge. The lack of universally accepted protocols can lead to inconsistencies in battery performance evaluation and quality control processes.
Lastly, the cost-effectiveness of implementing advanced acid concentration management systems in large-scale battery production is a significant hurdle. While sophisticated monitoring and control technologies exist, their integration into mass-produced batteries without substantially increasing costs remains a challenge for manufacturers.
Another significant challenge is the corrosive nature of battery acid, which can lead to degradation of battery components over time. This corrosion can affect the electrodes, separators, and other internal structures, ultimately impacting the battery's ability to maintain a stable voltage output. Researchers and manufacturers are continuously seeking ways to mitigate this corrosion while maintaining the necessary acid concentration for optimal performance.
The environmental impact of battery acid is also a pressing concern. Improper disposal or leakage of battery acid can have severe consequences for ecosystems and human health. This challenge extends beyond the technical aspects of battery performance and into the realm of environmental regulations and sustainable manufacturing practices.
Temperature sensitivity presents another hurdle in managing battery acid concentration. Extreme temperatures can affect the acid's concentration and reactivity, leading to variations in voltage output. This is particularly problematic in applications where batteries are exposed to wide temperature ranges, such as in automotive or outdoor energy storage systems.
The pursuit of higher energy densities in batteries also complicates the management of acid concentration. As manufacturers strive to pack more power into smaller battery packages, the balance between acid concentration and other battery components becomes increasingly delicate. This challenge requires innovative approaches to electrolyte design and cell architecture.
Standardization of measurement and control methods for acid concentration across different battery types and applications remains an ongoing challenge. The lack of universally accepted protocols can lead to inconsistencies in battery performance evaluation and quality control processes.
Lastly, the cost-effectiveness of implementing advanced acid concentration management systems in large-scale battery production is a significant hurdle. While sophisticated monitoring and control technologies exist, their integration into mass-produced batteries without substantially increasing costs remains a challenge for manufacturers.
Existing Solutions
01 Battery voltage measurement and monitoring
Systems and methods for accurately measuring and monitoring the voltage output of battery cells. This includes techniques for real-time voltage sensing, data acquisition, and analysis to assess battery health and performance.- Battery voltage measurement and monitoring: Systems and methods for accurately measuring and monitoring the voltage output of battery cells. This includes techniques for real-time voltage tracking, data collection, and analysis to assess battery performance and health.
- Battery management systems: Advanced battery management systems that control and optimize battery cell voltage output. These systems incorporate intelligent algorithms for balancing cell voltages, managing charge/discharge cycles, and enhancing overall battery efficiency and lifespan.
- Voltage regulation and stabilization: Technologies for regulating and stabilizing battery cell voltage output. This includes methods for maintaining consistent voltage levels, compensating for voltage fluctuations, and ensuring stable power delivery across various operating conditions.
- Battery cell voltage prediction and estimation: Techniques for predicting and estimating battery cell voltage output based on various parameters and historical data. These methods help in forecasting battery performance, remaining capacity, and potential voltage-related issues.
- Safety mechanisms for voltage control: Implementation of safety features and mechanisms to prevent overvoltage, undervoltage, and other voltage-related hazards in battery cells. This includes protective circuits, voltage limiters, and emergency shutdown systems to ensure safe operation of battery packs.
02 Battery management systems
Advanced battery management systems that control and optimize battery cell voltage output. These systems incorporate intelligent algorithms for balancing cell voltages, managing charge/discharge cycles, and ensuring safe operation of battery packs.Expand Specific Solutions03 Voltage regulation and stabilization
Techniques for regulating and stabilizing battery cell voltage output. This includes methods for maintaining consistent voltage levels, compensating for temperature variations, and minimizing voltage fluctuations during operation.Expand Specific Solutions04 Battery cell voltage prediction and estimation
Algorithms and models for predicting and estimating battery cell voltage output. These methods utilize historical data, operating conditions, and advanced mathematical models to forecast voltage behavior and remaining battery life.Expand Specific Solutions05 Safety mechanisms for voltage control
Safety features and mechanisms designed to prevent overcharging, over-discharging, and other voltage-related issues in battery cells. This includes protective circuits, voltage limiters, and emergency shutdown systems to ensure safe operation of battery-powered devices.Expand Specific Solutions
Key Industry Players
The impact of battery acid concentration on cell voltage output is a critical area of research in the battery industry, currently in a mature development stage. The market for battery technologies is substantial and growing, driven by increasing demand for energy storage solutions. Major players like Robert Bosch GmbH, DENSO Corp., and GS Yuasa International Ltd. are investing heavily in research and development to optimize battery performance. The technology is well-established, with companies such as Daramic LLC and Tianneng Battery Group Co., Ltd. focusing on improving separator materials and electrolyte formulations. Emerging players like Ningde Amperex Technology Ltd. are pushing boundaries in lithium-ion battery technology, while traditional lead-acid battery manufacturers continue to innovate in their field.
Robert Bosch GmbH
Technical Solution: Bosch has developed innovative solutions to address the impact of battery acid concentration on cell voltage output. Their approach includes the implementation of Enhanced Flooded Battery (EFB) technology, which incorporates a specialized polyester scrim to improve acid circulation and prevent stratification[10]. This ensures more uniform acid concentration across cells, leading to consistent voltage output. Bosch has also developed advanced grid designs that optimize current distribution and minimize local variations in acid concentration, contributing to improved overall battery performance[11]. Furthermore, the company has invested in smart charging technologies that adapt to the battery's state of charge and acid concentration, helping to maintain optimal voltage output throughout the battery's lifecycle[12].
Strengths: Innovative EFB technology, advanced grid designs, and smart charging solutions. Weaknesses: Potential limitations in extreme operating conditions and higher initial costs.
GS Yuasa International Ltd.
Technical Solution: GS Yuasa has developed advanced lead-acid battery technologies that optimize acid concentration for improved voltage output. Their proprietary Active Material (AM) technology enhances the electrochemical reactions within the battery, allowing for better utilization of the acid electrolyte[1]. This results in higher voltage stability and increased energy density. The company has also implemented a Valve Regulated Lead Acid (VRLA) design with optimized electrolyte distribution, ensuring consistent acid concentration across cells for uniform voltage output[2]. Additionally, GS Yuasa employs advanced grid alloys that resist corrosion and maintain structural integrity in high-acid environments, contributing to longer battery life and stable voltage performance[3].
Strengths: Improved energy density, longer battery life, and stable voltage output. Weaknesses: Higher production costs and potential limitations in extreme temperature conditions.
Core Innovations
Method for detecting battery stratification
PatentInactiveUS20080007225A1
Innovation
- An energy management algorithm that detects stratification by monitoring state of charge, working capacity, and voltage differential thresholds, and corrects it by increasing the battery charge voltage to remix the acid concentration.
Multi-region battery separators
PatentInactiveUS20170092917A1
Innovation
- A 3-region battery separator with a middle fiber region of specified thickness containing coarse fibers and peripheral regions of fine fibers, optionally with silica, is designed to enhance acid filling and plate formation by maintaining uniform acid concentration and resistance against acid stratification.
Environmental Impact
The environmental impact of battery acid concentration on cell voltage output is a critical consideration in the development and use of battery technologies. As the demand for energy storage solutions continues to grow, understanding and mitigating the environmental consequences of battery production and disposal becomes increasingly important.
The concentration of battery acid, typically sulfuric acid in lead-acid batteries, plays a significant role in determining the overall environmental footprint of these energy storage devices. Higher acid concentrations generally lead to improved battery performance and increased voltage output. However, this comes at a cost to the environment in several ways.
Firstly, the production of concentrated sulfuric acid requires substantial energy input and generates greenhouse gas emissions. As battery manufacturers strive for higher voltage outputs, the demand for more concentrated acid solutions increases, potentially exacerbating the carbon footprint associated with battery production.
Additionally, the disposal of batteries with higher acid concentrations poses greater environmental risks. When improperly discarded, these batteries can leak concentrated acid into soil and water systems, causing severe ecological damage. The acidity can disrupt local ecosystems, harm plant and animal life, and contaminate groundwater resources.
The recycling process for batteries with higher acid concentrations also presents challenges. While recycling is crucial for reducing the environmental impact of batteries, handling more concentrated acids requires additional safety measures and energy-intensive neutralization processes. This can increase the overall environmental cost of battery recycling efforts.
Furthermore, the transportation of batteries with higher acid concentrations poses increased safety risks and requires more stringent regulations. Accidental spills or leaks during transport can have severe environmental consequences, particularly in sensitive ecosystems or populated areas.
On the other hand, advancements in battery technology that allow for higher voltage outputs with lower acid concentrations could potentially reduce these environmental risks. Research into alternative electrolytes or battery chemistries that maintain high performance while minimizing the use of harmful acids is an important area of focus for sustainable battery development.
In conclusion, while higher battery acid concentrations can lead to improved cell voltage output, the environmental trade-offs must be carefully considered. Balancing performance requirements with environmental sustainability remains a key challenge for the battery industry. Future innovations in this field should aim to optimize voltage output while minimizing the ecological impact of battery production, use, and disposal.
The concentration of battery acid, typically sulfuric acid in lead-acid batteries, plays a significant role in determining the overall environmental footprint of these energy storage devices. Higher acid concentrations generally lead to improved battery performance and increased voltage output. However, this comes at a cost to the environment in several ways.
Firstly, the production of concentrated sulfuric acid requires substantial energy input and generates greenhouse gas emissions. As battery manufacturers strive for higher voltage outputs, the demand for more concentrated acid solutions increases, potentially exacerbating the carbon footprint associated with battery production.
Additionally, the disposal of batteries with higher acid concentrations poses greater environmental risks. When improperly discarded, these batteries can leak concentrated acid into soil and water systems, causing severe ecological damage. The acidity can disrupt local ecosystems, harm plant and animal life, and contaminate groundwater resources.
The recycling process for batteries with higher acid concentrations also presents challenges. While recycling is crucial for reducing the environmental impact of batteries, handling more concentrated acids requires additional safety measures and energy-intensive neutralization processes. This can increase the overall environmental cost of battery recycling efforts.
Furthermore, the transportation of batteries with higher acid concentrations poses increased safety risks and requires more stringent regulations. Accidental spills or leaks during transport can have severe environmental consequences, particularly in sensitive ecosystems or populated areas.
On the other hand, advancements in battery technology that allow for higher voltage outputs with lower acid concentrations could potentially reduce these environmental risks. Research into alternative electrolytes or battery chemistries that maintain high performance while minimizing the use of harmful acids is an important area of focus for sustainable battery development.
In conclusion, while higher battery acid concentrations can lead to improved cell voltage output, the environmental trade-offs must be carefully considered. Balancing performance requirements with environmental sustainability remains a key challenge for the battery industry. Future innovations in this field should aim to optimize voltage output while minimizing the ecological impact of battery production, use, and disposal.
Safety Regulations
Safety regulations play a crucial role in the handling and management of battery acid concentrations, particularly in the context of their impact on cell voltage output. These regulations are designed to protect workers, the environment, and the general public from potential hazards associated with battery acid exposure and manipulation.
One of the primary safety concerns addressed by regulations is the proper storage and handling of battery acid. Facilities dealing with battery acid must adhere to strict guidelines regarding containment, labeling, and storage conditions. This includes using corrosion-resistant containers, implementing proper ventilation systems, and maintaining appropriate temperature controls to prevent accidental spills or releases.
Personal protective equipment (PPE) requirements are another critical aspect of safety regulations. Workers involved in handling battery acid or working with batteries must wear appropriate PPE, including chemical-resistant gloves, goggles, face shields, and protective clothing. These measures are essential to minimize the risk of skin contact or inhalation of acid fumes, which can cause severe burns and respiratory issues.
Emergency response protocols are also mandated by safety regulations. Facilities must have clearly defined procedures for dealing with acid spills, including the availability of neutralizing agents, eyewash stations, and safety showers. Regular training sessions for employees on proper handling techniques and emergency response are typically required to ensure compliance with these regulations.
Environmental protection is a significant focus of safety regulations related to battery acid concentration. Strict guidelines govern the disposal of spent batteries and acid waste to prevent soil and water contamination. Recycling programs and proper neutralization processes are often mandated to minimize environmental impact and promote sustainable practices in the battery industry.
Workplace monitoring and regular safety inspections are integral components of regulatory compliance. This includes routine checks of acid concentration levels, pH monitoring, and assessment of storage conditions. Documentation of these checks and any incidents or near-misses is typically required for regulatory reporting and continuous improvement of safety protocols.
Transportation of battery acid is subject to specific regulations due to its corrosive nature. These regulations cover packaging requirements, labeling standards, and transportation methods to ensure safe movement of acid between facilities or to disposal sites. Compliance with these transportation regulations is essential to prevent accidents during transit and protect both handlers and the general public.
As technology advances and our understanding of the risks associated with battery acid improves, safety regulations continue to evolve. Regular updates to these regulations reflect new research findings, improved safety technologies, and changing industry practices. Staying informed about these regulatory changes is crucial for businesses operating in the battery sector to maintain compliance and ensure the highest standards of safety in their operations.
One of the primary safety concerns addressed by regulations is the proper storage and handling of battery acid. Facilities dealing with battery acid must adhere to strict guidelines regarding containment, labeling, and storage conditions. This includes using corrosion-resistant containers, implementing proper ventilation systems, and maintaining appropriate temperature controls to prevent accidental spills or releases.
Personal protective equipment (PPE) requirements are another critical aspect of safety regulations. Workers involved in handling battery acid or working with batteries must wear appropriate PPE, including chemical-resistant gloves, goggles, face shields, and protective clothing. These measures are essential to minimize the risk of skin contact or inhalation of acid fumes, which can cause severe burns and respiratory issues.
Emergency response protocols are also mandated by safety regulations. Facilities must have clearly defined procedures for dealing with acid spills, including the availability of neutralizing agents, eyewash stations, and safety showers. Regular training sessions for employees on proper handling techniques and emergency response are typically required to ensure compliance with these regulations.
Environmental protection is a significant focus of safety regulations related to battery acid concentration. Strict guidelines govern the disposal of spent batteries and acid waste to prevent soil and water contamination. Recycling programs and proper neutralization processes are often mandated to minimize environmental impact and promote sustainable practices in the battery industry.
Workplace monitoring and regular safety inspections are integral components of regulatory compliance. This includes routine checks of acid concentration levels, pH monitoring, and assessment of storage conditions. Documentation of these checks and any incidents or near-misses is typically required for regulatory reporting and continuous improvement of safety protocols.
Transportation of battery acid is subject to specific regulations due to its corrosive nature. These regulations cover packaging requirements, labeling standards, and transportation methods to ensure safe movement of acid between facilities or to disposal sites. Compliance with these transportation regulations is essential to prevent accidents during transit and protect both handlers and the general public.
As technology advances and our understanding of the risks associated with battery acid improves, safety regulations continue to evolve. Regular updates to these regulations reflect new research findings, improved safety technologies, and changing industry practices. Staying informed about these regulatory changes is crucial for businesses operating in the battery sector to maintain compliance and ensure the highest standards of safety in their operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!