How to Create Hypertonic Solutions for Biotechnological Applications?
Hypertonic Solutions in Biotech: Background and Objectives
Hypertonic solutions have played a crucial role in biotechnological applications for decades, serving as essential tools in various research and industrial processes. These solutions, characterized by their higher solute concentration compared to the surrounding environment, have become indispensable in fields such as cell biology, molecular biology, and bioengineering. The development of hypertonic solutions has evolved alongside advancements in biotechnology, with researchers continually refining their composition and applications to meet the growing demands of the industry.
The history of hypertonic solutions in biotechnology can be traced back to early studies on osmosis and cell membrane permeability. As our understanding of cellular processes deepened, scientists recognized the potential of manipulating osmotic pressure to achieve specific biological outcomes. This realization led to the systematic exploration of hypertonic solutions as tools for cell preservation, protein extraction, and plasmid isolation, among other applications.
Over time, the complexity and specificity of hypertonic solutions have increased dramatically. From simple salt-based formulations to sophisticated multi-component mixtures, these solutions have been tailored to address a wide range of biotechnological challenges. The evolution of hypertonic solutions has been driven by the need for improved efficiency, reproducibility, and scalability in biotech processes.
Current trends in hypertonic solution development focus on enhancing their biocompatibility, reducing toxicity, and expanding their functional versatility. Researchers are exploring novel solutes and combinations that can maintain cellular integrity while facilitating specific molecular interactions. Additionally, there is a growing interest in developing "smart" hypertonic solutions that can respond to environmental cues or be externally controlled, opening up new possibilities for targeted and dynamic applications in biotechnology.
The objectives of creating hypertonic solutions for biotechnological applications are multifaceted. Primarily, these solutions aim to provide precise control over osmotic pressure in biological systems, enabling researchers to manipulate cellular environments with unprecedented accuracy. This control is crucial for optimizing conditions in various bioprocesses, from cell culture and fermentation to protein purification and nucleic acid isolation.
Furthermore, hypertonic solutions are being developed to enhance the efficiency and yield of biotechnological processes. By carefully adjusting solution composition, researchers seek to improve the extraction of valuable biomolecules, increase the stability of biological products, and extend the shelf life of cell-based materials. The ultimate goal is to create hypertonic solutions that not only meet current industry standards but also push the boundaries of what is possible in biotechnology.
As we look to the future, the development of hypertonic solutions is expected to intersect with other emerging technologies, such as nanotechnology and artificial intelligence. This convergence promises to yield innovative approaches to solution design and application, potentially revolutionizing how we manipulate and study biological systems at the molecular level.
Market Analysis for Hypertonic Solutions in Biotechnology
The market for hypertonic solutions in biotechnology is experiencing significant growth, driven by the increasing demand for advanced bioprocessing techniques and the expanding applications in cell culture, cryopreservation, and tissue engineering. The global biotechnology market, which encompasses hypertonic solutions, was valued at $752.88 billion in 2020 and is projected to reach $2.44 trillion by 2028, with a compound annual growth rate (CAGR) of 15.83% during the forecast period.
Hypertonic solutions play a crucial role in various biotechnological applications, particularly in cell culture and preservation. The cell culture market, a key segment utilizing hypertonic solutions, is expected to grow from $21.1 billion in 2020 to $41.3 billion by 2026, with a CAGR of 11.8%. This growth is primarily attributed to the increasing demand for biopharmaceuticals and regenerative medicine.
The cryopreservation market, another significant area for hypertonic solutions, is forecasted to reach $7.7 billion by 2025, growing at a CAGR of 8.9% from 2020 to 2025. The rising need for cell and tissue banking, along with the growing focus on personalized medicine, is driving this market segment.
Geographically, North America dominates the market for hypertonic solutions in biotechnology, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to its advanced biotechnology infrastructure and significant investments in research and development. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the highest growth rates in the coming years.
Key players in the hypertonic solutions market include Thermo Fisher Scientific, Merck KGaA, Lonza Group, and Corning Incorporated. These companies are focusing on product innovation and strategic collaborations to maintain their market positions and meet the evolving needs of the biotechnology industry.
The increasing adoption of 3D cell culture techniques and the growing emphasis on personalized medicine are expected to create new opportunities for hypertonic solutions in biotechnology. Additionally, the rising demand for stem cell research and regenerative medicine is likely to further boost the market growth in the coming years.
However, challenges such as stringent regulatory requirements and the high cost of advanced bioprocessing technologies may hinder market growth to some extent. Despite these challenges, the overall market outlook for hypertonic solutions in biotechnology remains positive, driven by continuous technological advancements and the expanding applications in various biotechnological fields.
Current Challenges in Hypertonic Solution Development
The development of hypertonic solutions for biotechnological applications faces several significant challenges that hinder progress in this field. One of the primary obstacles is maintaining the stability of these solutions over extended periods. Hypertonic solutions often contain high concentrations of solutes, which can lead to precipitation or degradation of components over time, compromising their effectiveness and shelf life.
Another critical challenge lies in the precise control of osmolarity and tonicity. Achieving and maintaining the exact desired level of hypertonicity is crucial for many biotechnological applications, such as cell culture and cryopreservation. However, factors like temperature fluctuations and interactions between solution components can affect these properties, making it difficult to ensure consistent performance across different batches or storage conditions.
The biocompatibility of hypertonic solutions presents another significant hurdle. While these solutions need to be highly concentrated, they must also remain non-toxic and compatible with biological systems. Balancing the need for high solute concentrations with minimal cellular damage or stress responses is a complex task that requires extensive research and optimization.
Scalability in production is an additional challenge faced by researchers and manufacturers. Techniques that work well in small-scale laboratory settings may not translate effectively to large-scale industrial production. Issues such as mixing efficiency, heat transfer, and filtration become more pronounced at larger scales, necessitating the development of specialized equipment and processes.
Furthermore, the selection of appropriate solutes for specific applications poses its own set of challenges. Different biotechnological applications may require varying types of hypertonic solutions, each with its unique set of requirements. Finding solutes that not only provide the desired osmotic effects but also meet other criteria such as pH stability, chemical inertness, and cost-effectiveness can be a complex and time-consuming process.
Regulatory compliance adds another layer of complexity to hypertonic solution development. Stringent quality control measures and documentation requirements must be met, especially for solutions intended for clinical or pharmaceutical applications. This necessitates extensive testing and validation procedures, which can significantly extend development timelines and increase costs.
Lastly, the environmental impact of producing and disposing of hypertonic solutions is an emerging concern. As sustainability becomes increasingly important in biotechnology, developing eco-friendly formulations and production methods that minimize waste and energy consumption presents a new frontier of challenges for researchers in this field.
Existing Methods for Creating Hypertonic Solutions
01 Hypertonic solutions for medical applications
Hypertonic solutions are used in various medical applications due to their higher solute concentration compared to body fluids. These solutions can be used for treating conditions such as edema, increasing blood volume, and as a component in certain medical procedures. The tonicity of these solutions is carefully controlled to achieve the desired therapeutic effect.- Hypertonic solutions for medical applications: Hypertonic solutions are used in various medical applications due to their higher solute concentration compared to body fluids. These solutions can be used for treating conditions such as edema, increasing blood volume, and as a component in dialysis fluids. The tonicity of these solutions is carefully controlled to achieve the desired therapeutic effect while minimizing potential side effects.
- Tonicity adjustment in pharmaceutical formulations: Adjusting the tonicity of pharmaceutical formulations is crucial for ensuring their compatibility with biological systems. This process involves adding or modifying the concentration of solutes to achieve isotonicity or a specific level of hypertonicity. Tonicity agents such as sodium chloride, dextrose, or mannitol are commonly used for this purpose in various drug formulations and ophthalmic solutions.
- Hypertonic solutions in cell culture and biotechnology: Hypertonic solutions play a significant role in cell culture techniques and biotechnology applications. These solutions can be used to induce osmotic stress in cells, study cellular responses to hyperosmotic conditions, and optimize growth conditions for certain microorganisms. The careful manipulation of solution tonicity is essential for maintaining cell viability and achieving desired experimental outcomes.
- Measurement and control of solution tonicity: Accurate measurement and control of solution tonicity are critical in various fields, including medicine, pharmaceuticals, and biotechnology. Techniques such as osmometry, freezing point depression, and vapor pressure measurements are used to determine the tonicity of solutions. Advanced methods and devices have been developed to ensure precise control of solution tonicity in both research and industrial settings.
- Applications of hypertonic solutions in food and beverage industry: Hypertonic solutions find applications in the food and beverage industry for processes such as osmotic dehydration, preservation, and flavor enhancement. The use of hypertonic solutions in food processing can help extend shelf life, improve texture, and modify the sensory properties of various products. Understanding and controlling the tonicity of these solutions is crucial for achieving desired product characteristics and ensuring food safety.
02 Osmotic pressure regulation in cell culture
Hypertonic solutions play a crucial role in regulating osmotic pressure in cell culture media. By adjusting the tonicity of the culture environment, researchers can control cell growth, differentiation, and other cellular processes. This is particularly important in biotechnology and pharmaceutical research for optimizing cell-based production systems.Expand Specific Solutions03 Hypertonic solutions in ophthalmic formulations
Hypertonic solutions are utilized in ophthalmic formulations to treat various eye conditions. These solutions can help reduce corneal edema, manage intraocular pressure, and provide relief for dry eye syndrome. The tonicity of these formulations is carefully balanced to ensure effectiveness while minimizing ocular irritation.Expand Specific Solutions04 Tonicity adjustment in pharmaceutical preparations
The tonicity of pharmaceutical preparations is often adjusted using hypertonic solutions to ensure compatibility with biological systems. This is crucial for parenteral formulations, where the osmotic balance must be maintained to prevent adverse effects. Tonicity adjusters such as sodium chloride or dextrose are commonly used to achieve the desired osmolality.Expand Specific Solutions05 Hypertonic solutions in diagnostic applications
Hypertonic solutions are employed in various diagnostic applications, including contrast media for imaging studies and reagents for in vitro diagnostic tests. The tonicity of these solutions is carefully controlled to ensure optimal performance and compatibility with biological samples, while also maintaining the stability of the diagnostic components.Expand Specific Solutions
Key Players in Hypertonic Solution Industry
The creation of hypertonic solutions for biotechnological applications is a rapidly evolving field in the mature biotechnology industry. The market for these solutions is expanding, driven by increasing demand in pharmaceutical, research, and medical sectors. Companies like Medtronic, Genentech, and Novartis are at the forefront, leveraging their extensive R&D capabilities to develop advanced hypertonic solutions. Academic institutions such as MIT and University of California are contributing significant research, pushing the boundaries of technology maturity. The competitive landscape is characterized by a mix of established pharmaceutical giants and specialized biotech firms, with emerging players like Cellphire and DeCell Technologies introducing innovative approaches to hypertonic solution development.
Medtronic, Inc.
Genentech, Inc.
Innovative Approaches in Hypertonic Solution Formulation
- The use of a hypertonic solution, specifically a hypertonic salt solution with a salt concentration ranging from about 1% to 8%, is introduced to enhance the delivery of gene therapy vectors by contacting cells with it before, after, or simultaneously with the vectors, thereby improving gene transfer efficiency.
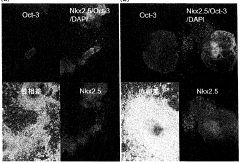
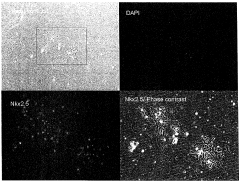
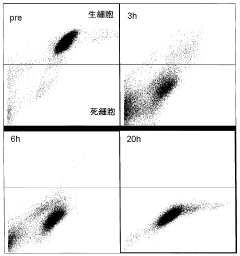
- A method involving culturing pluripotent stem cells and differentiated cells in a hypertonic solution with an osmotic pressure of 370 mOsm/kg or more, using carbohydrates like mannitol or glycerol, to induce cell death in undifferentiated cells and non-cardiomyocytes without affecting cardiomyocytes, thereby purifying cardiomyocytes.
Regulatory Considerations for Hypertonic Solutions in Biotech
The regulatory landscape for hypertonic solutions in biotechnological applications is complex and multifaceted, requiring careful consideration throughout the development and implementation process. These solutions, characterized by their higher solute concentration compared to physiological fluids, are subject to stringent oversight due to their potential impact on biological systems.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating hypertonic solutions used in biotech applications. The agency's approach varies depending on the intended use of the solution, with different regulatory pathways for pharmaceuticals, medical devices, and research tools. For pharmaceutical applications, hypertonic solutions may fall under the purview of the Center for Drug Evaluation and Research (CDER), necessitating rigorous clinical trials and safety assessments before approval.
The European Medicines Agency (EMA) oversees similar regulatory processes in the European Union, with additional considerations for harmonization across member states. The EMA's guidelines on quality, safety, and efficacy must be adhered to, particularly when hypertonic solutions are components of medicinal products or advanced therapy medicinal products (ATMPs).
Regulatory bodies worldwide emphasize the importance of Good Manufacturing Practices (GMP) in the production of hypertonic solutions for biotech applications. This includes stringent quality control measures, validated production processes, and comprehensive documentation. The International Conference on Harmonisation (ICH) guidelines provide a global framework for quality standards, which many national regulatory agencies have adopted.
Environmental considerations also factor into the regulatory landscape. The disposal of hypertonic solutions and their potential impact on ecosystems may be subject to environmental protection regulations. This is particularly relevant for large-scale industrial applications of hypertonic solutions in biotech processes.
For research applications, institutional review boards (IRBs) and ethics committees play a crucial role in overseeing the use of hypertonic solutions in experimental settings. These bodies ensure that research protocols adhere to ethical standards and minimize risks to human or animal subjects.
As biotechnology continues to advance, regulatory frameworks are evolving to keep pace with new applications of hypertonic solutions. This includes emerging fields such as cell therapy and tissue engineering, where hypertonic solutions may play critical roles in cell preservation or scaffold development. Regulatory agencies are increasingly adopting adaptive approaches to accommodate these innovations while maintaining rigorous safety and efficacy standards.
Environmental Impact of Hypertonic Solution Production
The production of hypertonic solutions for biotechnological applications has significant environmental implications that warrant careful consideration. The process often involves the use of various chemicals and resources, which can impact ecosystems if not managed properly. One primary concern is the disposal of waste products generated during the production process. These may include excess salts, organic compounds, and other additives that, if released untreated into water systems, can disrupt aquatic ecosystems and potentially harm wildlife.
Water consumption is another critical environmental factor in hypertonic solution production. The process typically requires large volumes of purified water, which can strain local water resources, especially in water-scarce regions. This increased demand for water may lead to competition with other essential uses, such as agriculture or domestic consumption, potentially exacerbating water scarcity issues in affected areas.
Energy usage in the production of hypertonic solutions also contributes to environmental concerns. The purification of water, sterilization processes, and the operation of production facilities all require significant energy inputs. Depending on the energy sources used, this can result in increased greenhouse gas emissions and contribute to climate change. Implementing energy-efficient technologies and transitioning to renewable energy sources can help mitigate these impacts.
The packaging and transportation of hypertonic solutions present additional environmental challenges. Single-use plastic containers, commonly used in biotechnology applications, contribute to plastic waste and pollution. Furthermore, the transportation of these solutions, often requiring temperature-controlled conditions, increases carbon emissions from vehicles and refrigeration units.
To address these environmental concerns, many biotechnology companies are adopting more sustainable practices. These include implementing closed-loop systems to recycle water and chemicals, utilizing biodegradable packaging materials, and optimizing production processes to reduce waste. Some facilities are also exploring the use of green chemistry principles to develop more environmentally friendly hypertonic solutions, using less harmful chemicals and reducing the overall environmental footprint of production.
Regulatory bodies are increasingly focusing on the environmental impact of biotechnological processes, including the production of hypertonic solutions. This has led to stricter guidelines for waste management, emissions control, and resource usage in the industry. Companies are now required to conduct environmental impact assessments and implement mitigation strategies to minimize their ecological footprint.
As the demand for hypertonic solutions in biotechnology continues to grow, balancing production needs with environmental stewardship becomes increasingly crucial. Ongoing research and development efforts are focused on finding more sustainable methods for creating these solutions, aiming to reduce environmental impacts while maintaining the high quality and efficacy required for biotechnological applications.