Hypertonic Nasal Sprays: Efficacy and Formulation Techniques
Hypertonic Nasal Spray Evolution and Objectives
Hypertonic nasal sprays have emerged as a significant advancement in the field of nasal care and respiratory health. The evolution of these sprays can be traced back to the early understanding of osmosis and its effects on biological membranes. Initially, saline solutions were used for nasal irrigation, but the concept of hypertonic solutions for nasal application gained traction in the late 20th century.
The development of hypertonic nasal sprays was driven by the need for more effective treatments for nasal congestion, allergies, and sinus-related issues. Traditional isotonic saline sprays, while beneficial, had limitations in their ability to reduce mucosal edema and improve mucus clearance. Researchers began exploring the potential of higher salt concentrations to enhance these effects.
The 1990s saw a surge in clinical studies investigating the efficacy of hypertonic saline solutions for various nasal conditions. These studies laid the groundwork for the formulation of commercial hypertonic nasal sprays. The primary objective was to create a product that could effectively reduce nasal congestion, improve mucociliary clearance, and provide symptomatic relief without the side effects associated with pharmacological decongestants.
As the technology progressed, formulation techniques became more sophisticated. The focus shifted from simple saline solutions to more complex formulations incorporating additional ingredients such as xylitol, essential oils, and preservatives. These advancements aimed to enhance the spray's effectiveness, improve its sensory properties, and extend its shelf life.
The objectives of modern hypertonic nasal spray development extend beyond symptom relief. Current research aims to optimize formulations for specific conditions, such as chronic rhinosinusitis, allergic rhinitis, and even as an adjunct therapy for upper respiratory infections. There is also a growing interest in exploring the potential of hypertonic sprays as delivery vehicles for other therapeutic agents.
Another key objective in the evolution of hypertonic nasal sprays is to improve patient compliance and ease of use. This has led to innovations in spray bottle design, focusing on factors such as spray pattern, droplet size, and dose consistency. The goal is to ensure effective delivery of the solution to the nasal mucosa while minimizing discomfort and improving the overall user experience.
Looking forward, the objectives for hypertonic nasal spray development include further refinement of formulations to maximize efficacy while minimizing potential irritation. There is also a push towards more personalized solutions, tailoring the tonicity and composition of sprays to individual patient needs. Additionally, research is ongoing to explore the potential of hypertonic sprays in new therapeutic areas, such as enhancing the nasal microbiome or as a preventive measure against airborne pathogens.
Market Analysis for Nasal Decongestant Solutions
The global market for nasal decongestant solutions has been experiencing steady growth, driven by increasing prevalence of allergies, sinusitis, and other respiratory conditions. Hypertonic nasal sprays, in particular, have gained significant attention due to their efficacy in providing relief from nasal congestion and their potential for improved formulation techniques.
The market for nasal decongestants is segmented based on product types, including saline solutions, corticosteroids, antihistamines, and decongestants. Hypertonic saline solutions have emerged as a popular choice among consumers due to their natural composition and minimal side effects. This segment is expected to witness substantial growth in the coming years as more consumers seek non-medicated alternatives for nasal congestion relief.
Geographically, North America and Europe dominate the nasal decongestant market, owing to high awareness levels, advanced healthcare infrastructure, and a large patient pool. However, the Asia-Pacific region is projected to exhibit the highest growth rate, attributed to rising disposable incomes, increasing healthcare expenditure, and growing awareness about respiratory health.
Key market players in the nasal decongestant space include GlaxoSmithKline, Johnson & Johnson, Pfizer, and Procter & Gamble. These companies are investing heavily in research and development to improve formulation techniques and enhance the efficacy of their products. The focus on developing hypertonic nasal sprays with improved delivery systems and longer-lasting effects is a notable trend in the industry.
Consumer preferences are shifting towards natural and organic ingredients, prompting manufacturers to explore plant-based formulations and eco-friendly packaging options. This trend is expected to drive innovation in the hypertonic nasal spray segment, with companies developing products that cater to health-conscious consumers.
The COVID-19 pandemic has had a significant impact on the nasal decongestant market, with increased demand for products that provide relief from respiratory symptoms. This has led to a surge in sales of nasal sprays and other decongestant solutions, particularly those with antiviral properties.
Looking ahead, the market for nasal decongestant solutions, especially hypertonic nasal sprays, is poised for continued growth. Factors such as increasing air pollution, rising incidence of allergies, and growing awareness about nasal hygiene are expected to drive market expansion. Additionally, the development of novel formulation techniques and delivery systems will likely enhance the efficacy and user experience of hypertonic nasal sprays, further boosting their market potential.
Current Challenges in Hypertonic Nasal Spray Development
The development of hypertonic nasal sprays faces several significant challenges that hinder their widespread adoption and efficacy. One of the primary obstacles is achieving the optimal balance between tonicity and tolerability. While higher salt concentrations can potentially enhance the therapeutic effects, they may also cause discomfort and irritation to the nasal mucosa, leading to poor patient compliance.
Formulation stability presents another major hurdle. Hypertonic solutions are inherently prone to crystallization, especially during storage and temperature fluctuations. This can affect the spray's consistency, dosage accuracy, and overall shelf life. Manufacturers must develop innovative stabilization techniques to maintain the integrity of the formulation over extended periods.
The delivery mechanism poses additional challenges. Ensuring uniform and consistent droplet size distribution is crucial for effective deposition in the nasal cavity. However, the high viscosity of hypertonic solutions can interfere with spray patterns and droplet formation, potentially reducing the spray's efficacy and patient comfort during administration.
Preservative compatibility is another area of concern. Many traditional preservatives used in nasal sprays may not be as effective in hypertonic environments, necessitating the development of alternative preservation systems that can maintain product sterility without compromising safety or efficacy.
Regulatory hurdles also present significant challenges. As hypertonic nasal sprays often straddle the line between medical devices and drug products, navigating the regulatory landscape can be complex. Manufacturers must address varying requirements across different regions, which can impact formulation choices and clinical trial designs.
The potential for long-term effects on nasal physiology remains a subject of ongoing research. While short-term benefits are well-documented, the impact of prolonged use of hypertonic sprays on nasal mucosa and ciliary function is not fully understood. This gap in knowledge necessitates extensive long-term safety studies.
Lastly, patient education and adherence pose significant challenges. The proper technique for using hypertonic nasal sprays is crucial for their effectiveness, yet many patients struggle with correct administration. Developing user-friendly devices and comprehensive patient education programs is essential to maximize therapeutic outcomes and minimize adverse effects.
Existing Formulation Techniques for Hypertonic Nasal Sprays
01 Composition of hypertonic nasal sprays
Hypertonic nasal sprays typically contain a higher concentration of salt (sodium chloride) than found in body cells. This creates an osmotic gradient that helps draw fluid out of the nasal tissues, reducing inflammation and congestion. Some formulations may also include additional ingredients such as preservatives, buffering agents, or moisturizers to enhance efficacy and comfort.- Composition of hypertonic nasal sprays: Hypertonic nasal sprays typically contain a higher concentration of salt (sodium chloride) than found in body cells. This creates an osmotic gradient that helps draw water out of the nasal tissues, reducing inflammation and congestion. Some formulations may also include additional ingredients such as preservatives, buffering agents, or moisturizers to enhance efficacy and comfort.
- Efficacy in treating nasal congestion and rhinitis: Hypertonic nasal sprays have shown effectiveness in alleviating symptoms of nasal congestion and rhinitis. The high salt concentration helps to reduce mucosal edema, improve mucus clearance, and decrease inflammatory mediators. This can lead to improved breathing, reduced nasal discharge, and overall symptom relief for conditions such as allergic rhinitis and sinusitis.
- Delivery methods and devices: Various delivery methods and devices have been developed to improve the efficacy of hypertonic nasal sprays. These include specialized nozzles designed for optimal distribution within the nasal cavity, metered-dose systems for accurate dosing, and combination devices that may incorporate additional features such as nasal irrigation or drug delivery capabilities.
- Adjunct therapy and combination products: Hypertonic nasal sprays are often used as adjunct therapy or in combination with other treatments. Some formulations may include additional active ingredients such as decongestants, antihistamines, or corticosteroids to enhance their therapeutic effects. These combination products aim to provide comprehensive relief from various nasal and sinus symptoms.
- Long-term safety and efficacy studies: Research has been conducted to evaluate the long-term safety and efficacy of hypertonic nasal sprays. Studies have examined factors such as optimal concentration, frequency of use, and potential side effects. Results generally support the safety and effectiveness of these products for extended use in managing chronic nasal conditions, with minimal adverse effects reported.
02 Efficacy in treating nasal congestion and rhinitis
Hypertonic nasal sprays have shown effectiveness in alleviating symptoms of nasal congestion and rhinitis. The high salt concentration helps to reduce mucosal edema, improve mucus clearance, and enhance ciliary function. This can lead to improved breathing, reduced inflammation, and relief from nasal symptoms associated with various conditions, including allergies and sinusitis.Expand Specific Solutions03 Delivery methods and devices
Various delivery methods and devices have been developed to optimize the application of hypertonic nasal sprays. These include specially designed spray bottles, nebulizers, and nasal irrigation systems. Some devices focus on providing a fine mist for even distribution, while others offer controlled dosing or pulsatile delivery to enhance efficacy and user comfort.Expand Specific Solutions04 Combination with other active ingredients
To enhance the therapeutic effects, some hypertonic nasal spray formulations incorporate additional active ingredients. These may include antimicrobial agents, decongestants, or anti-inflammatory compounds. The combination of hypertonic saline with these additives can potentially offer broader spectrum relief and improved efficacy in managing various nasal conditions.Expand Specific Solutions05 Safety and long-term use considerations
Studies have investigated the safety profile and long-term effects of hypertonic nasal sprays. Generally, they are considered safe for regular use, with minimal side effects. However, some formulations may cause temporary discomfort or irritation in certain individuals. Research has also explored the impact of prolonged use on nasal mucosa and ciliary function to ensure the overall safety and efficacy of these products.Expand Specific Solutions
Key Players in Nasal Spray Pharmaceutical Industry
The hypertonic nasal spray market is in a growth phase, driven by increasing demand for non-invasive drug delivery methods and rising respiratory disorders. The global market size is projected to expand significantly in the coming years. Technologically, the field is advancing rapidly, with companies like Glaxo Group Ltd., Novartis Vaccines & Diagnostics Srl, and Pfizer Inc. leading innovation in formulation techniques. Emerging players such as Sensory Cloud, Inc. and ARS Pharmaceuticals Operations, Inc. are also contributing to technological advancements. The competitive landscape is diverse, with pharmaceutical giants, specialized biotech firms, and academic institutions like the University of Washington and Johns Hopkins University collaborating to improve efficacy and develop novel formulations for hypertonic nasal sprays.
Glaxo Group Ltd.
Aegis Therapeutics LLC
Innovative Approaches in Hypertonic Solution Stability
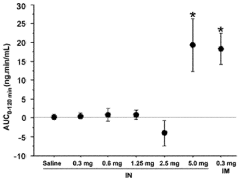
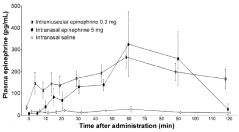
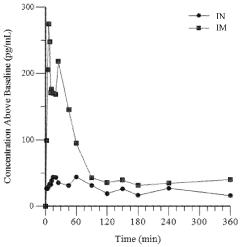
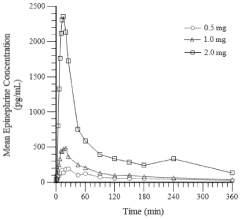
- Development of an intranasal epinephrine formulation in a compact, needle-free nasal spray device providing consistent and rapid administration, with formulations containing between 0.40 mg to 2.4 mg of epinephrine, designed to achieve intramuscular-like pharmacokinetics for effective treatment of anaphylaxis and other conditions.
- A pharmaceutical composition comprising cannabidiol and glatiramer acetate, or other active ingredients, combined with a carrier system of water, C2-C4 alcohols (preferably ethanol), and phospholipids in a vesicular structure, along with a polyol like propylene glycol, optimized for intranasal administration to enhance bioavailability and therapeutic efficacy.
Regulatory Framework for OTC Nasal Spray Products
The regulatory framework for over-the-counter (OTC) nasal spray products is a complex and evolving landscape that manufacturers must navigate to ensure compliance and market access. In the United States, the Food and Drug Administration (FDA) oversees the regulation of OTC nasal sprays, classifying them as drug products. The FDA's monograph system provides guidelines for active ingredients, dosage forms, and labeling requirements for OTC nasal sprays.
Under the OTC monograph system, manufacturers can market their products without prior FDA approval if they adhere to the established monograph guidelines. However, for novel formulations or active ingredients not covered by existing monographs, manufacturers must submit a New Drug Application (NDA) for FDA review and approval.
The FDA's regulations for OTC nasal sprays encompass several key areas. These include Good Manufacturing Practices (GMP) to ensure product quality and consistency, stability testing requirements to determine shelf life, and packaging and labeling regulations to provide clear and accurate information to consumers.
In the European Union, the regulatory framework for OTC nasal sprays falls under the jurisdiction of the European Medicines Agency (EMA) and national regulatory authorities. The EU employs a harmonized approach through the Mutual Recognition Procedure (MRP) and Decentralized Procedure (DCP) for marketing authorization across member states.
Japan's regulatory framework for OTC nasal sprays is overseen by the Pharmaceuticals and Medical Devices Agency (PMDA). The Japanese system categorizes OTC drugs into different classes based on risk levels, with specific regulatory requirements for each class.
Globally, regulatory bodies are increasingly focusing on the safety and efficacy of preservatives used in nasal spray formulations. This has led to stricter guidelines on the use of certain preservatives and a push towards preservative-free formulations where possible.
Manufacturers must also consider specific regulations for hypertonic nasal sprays, which may be classified differently from isotonic formulations due to their higher salt concentration. These regulations may include additional safety assessments and specific labeling requirements to inform consumers about potential effects on nasal mucosa.
As the field of nasal drug delivery continues to advance, regulatory frameworks are adapting to address new technologies and formulation techniques. This includes considerations for novel delivery devices, nanotechnology-based formulations, and combination products that may incorporate both drug and device components.
Patient Compliance and User Experience Considerations
Patient compliance and user experience are critical factors in the success of hypertonic nasal sprays. The effectiveness of these sprays relies heavily on proper and consistent use by patients. To ensure optimal outcomes, manufacturers must consider several key aspects of patient compliance and user experience in their formulation and device design processes.
One of the primary considerations is the ease of use of the nasal spray device. The design should be intuitive, allowing patients to administer the correct dose with minimal effort or confusion. Ergonomic factors, such as the shape and size of the bottle, the positioning of the nozzle, and the pressure required to activate the spray, all play crucial roles in user experience. A well-designed device can significantly improve patient adherence to the prescribed treatment regimen.
The sensory experience of using the nasal spray is another important factor. Hypertonic solutions can sometimes cause discomfort or irritation in the nasal passages. Formulation techniques that minimize these side effects while maintaining efficacy are essential. This may involve the inclusion of soothing agents or the optimization of particle size to ensure even distribution within the nasal cavity. The goal is to create a product that patients find comfortable to use, thereby encouraging regular application.
The frequency of administration is a key consideration in patient compliance. Formulations that require less frequent application may lead to better adherence, as patients are more likely to follow a simpler regimen. However, this must be balanced with the need for sustained therapeutic effects. Innovative formulation techniques, such as the use of mucoadhesive agents or controlled-release systems, may help extend the duration of action and reduce the required frequency of use.
Clear and concise instructions are vital for proper use and patient compliance. Package inserts, labeling, and even digital resources should provide easily understandable guidance on how to use the nasal spray correctly. This includes information on proper technique, dosage, and frequency of use. Some manufacturers have explored the use of smart devices or mobile applications to provide interactive instructions and reminders, further supporting patient adherence.
The storage and portability of the nasal spray can also impact user experience and compliance. Formulations that remain stable at room temperature and do not require refrigeration are generally more convenient for patients. Additionally, compact and durable packaging designs that allow for easy transport can encourage patients to keep the medication with them, promoting consistent use even when away from home.
Lastly, the overall efficacy of the product in providing symptom relief plays a significant role in patient compliance. Patients are more likely to adhere to a treatment regimen if they experience noticeable benefits. Therefore, formulation techniques that enhance the therapeutic effects of hypertonic nasal sprays, such as optimizing the concentration of saline or incorporating complementary active ingredients, can indirectly improve compliance through increased patient satisfaction.