Hypertonic Fluids: Enhancing Drug Delivery Systems
Hypertonic Fluids Background and Objectives
Hypertonic fluids have emerged as a promising avenue in the field of drug delivery systems, offering potential solutions to enhance the efficacy and precision of therapeutic interventions. The concept of utilizing hypertonic solutions to improve drug delivery has its roots in the fundamental principles of osmosis and cellular physiology. Over the past few decades, researchers have increasingly focused on exploiting the unique properties of hypertonic fluids to overcome various barriers in drug administration.
The evolution of hypertonic fluid technology in drug delivery can be traced back to early observations of osmotic effects on cellular membranes. As our understanding of cell biology and pharmacokinetics advanced, scientists began to explore how manipulating the osmotic environment could influence drug uptake and distribution. This led to the development of various hypertonic formulations designed to enhance drug penetration across biological barriers.
The primary objective of research in this field is to leverage the osmotic gradient created by hypertonic fluids to facilitate more efficient drug delivery. By creating an environment where the concentration of solutes outside the cell is higher than inside, researchers aim to induce osmotic flow that can carry drug molecules more effectively into target tissues. This approach holds particular promise for overcoming challenges associated with the blood-brain barrier, ocular drug delivery, and transdermal applications.
Recent technological advancements have further expanded the potential of hypertonic fluids in drug delivery systems. Innovations in nanoparticle formulations, smart polymers, and controlled release mechanisms have opened new avenues for integrating hypertonic solutions into sophisticated drug delivery platforms. These developments aim to achieve greater specificity in targeting, improved bioavailability, and reduced systemic side effects.
The current research landscape is focused on optimizing the composition and application of hypertonic fluids to maximize their benefits while minimizing potential drawbacks. Key areas of investigation include the development of biocompatible hypertonic solutions, fine-tuning of osmolarity levels for specific therapeutic applications, and exploring synergistic effects with other drug delivery technologies. Additionally, researchers are examining the potential of hypertonic fluids in combination with emerging fields such as gene therapy and immunotherapy.
As we look towards the future, the goals of hypertonic fluid research in drug delivery systems are multifaceted. Scientists aim to develop more targeted and efficient delivery methods, reduce drug dosages while maintaining therapeutic efficacy, and expand the range of drugs that can benefit from this approach. There is also a growing emphasis on translating laboratory findings into clinically viable treatments, addressing regulatory challenges, and ensuring the safety and efficacy of hypertonic fluid-based drug delivery systems in diverse patient populations.
Market Analysis for Advanced Drug Delivery Systems
The advanced drug delivery systems market has been experiencing significant growth, driven by the increasing demand for targeted and controlled release of therapeutic agents. This market segment is expected to continue its upward trajectory due to the rising prevalence of chronic diseases, the need for more effective treatments, and the growing emphasis on personalized medicine.
Hypertonic fluids, as a novel approach in drug delivery systems, are gaining traction due to their potential to enhance the efficacy and bioavailability of various medications. The market for hypertonic fluid-based drug delivery systems is still in its nascent stage but shows promising growth prospects. This emerging technology is attracting attention from both pharmaceutical companies and healthcare providers due to its ability to improve drug absorption and distribution within the body.
The global market for advanced drug delivery systems is highly competitive, with key players including Johnson & Johnson, Pfizer, Novartis, and Merck & Co. These companies are investing heavily in research and development to stay ahead in the rapidly evolving landscape. Smaller biotech firms and startups are also making significant contributions to the field, often focusing on niche applications or specific therapeutic areas.
Geographically, North America and Europe currently dominate the advanced drug delivery systems market, owing to their well-established healthcare infrastructure, high R&D investments, and favorable regulatory environments. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness about advanced medical technologies, and improving access to healthcare services.
The market for hypertonic fluid-based drug delivery systems is particularly promising in oncology, where targeted drug delivery is crucial for improving treatment outcomes and reducing side effects. Other therapeutic areas showing potential include neurology, ophthalmology, and cardiovascular diseases. The ability of hypertonic fluids to enhance drug penetration through biological barriers makes them especially valuable in these fields.
Despite the positive outlook, the market faces several challenges. Regulatory hurdles, high development costs, and the need for extensive clinical trials can slow down the commercialization of new drug delivery technologies. Additionally, concerns about potential side effects and long-term safety of hypertonic fluid-based systems need to be addressed through rigorous research and clinical studies.
In conclusion, the market for advanced drug delivery systems, particularly those utilizing hypertonic fluids, presents significant opportunities for growth and innovation. As research in this field progresses and more applications are discovered, we can expect to see increased adoption of these technologies across various therapeutic areas, ultimately leading to improved patient outcomes and a transformation in the way medications are delivered and administered.
Current Challenges in Hypertonic Fluid Technology
Despite the promising potential of hypertonic fluids in drug delivery systems, several significant challenges persist in their development and application. One of the primary obstacles is the difficulty in maintaining the stability of hypertonic solutions over extended periods. The high solute concentration can lead to precipitation or crystallization of components, potentially altering the fluid's properties and compromising its efficacy.
Another critical challenge lies in controlling the osmotic effects of hypertonic fluids on biological tissues. While the osmotic gradient is crucial for enhancing drug penetration, excessive osmotic pressure can cause tissue damage or irritation, particularly in sensitive areas such as mucosal membranes. Balancing the osmotic strength to maximize drug delivery while minimizing adverse effects remains a complex task.
The biocompatibility of hypertonic fluids also presents ongoing concerns. Some solutes used to create hypertonic solutions may trigger immune responses or cause local inflammation, limiting their applicability in certain patient populations or for specific therapeutic purposes. Developing hypertonic formulations that are both effective and well-tolerated by the body is an ongoing challenge for researchers.
Furthermore, the interaction between hypertonic fluids and various drug molecules poses significant hurdles. Some drugs may exhibit altered solubility, stability, or release kinetics in hypertonic environments, potentially affecting their therapeutic efficacy. Understanding and optimizing these complex interactions for a wide range of pharmaceutical compounds remains a considerable challenge in the field.
The scalability of hypertonic fluid production for commercial applications is another area of concern. Ensuring consistent quality and maintaining the precise osmolality of large-scale batches can be technically challenging and cost-intensive. This aspect is particularly crucial for the pharmaceutical industry, where stringent quality control measures are essential.
Additionally, the regulatory landscape surrounding hypertonic fluids in drug delivery systems is still evolving. Establishing clear guidelines and standardized testing protocols for these novel formulations can be complex, potentially slowing down the approval process for new hypertonic fluid-based drug delivery systems.
Lastly, there is a need for more comprehensive in vivo studies to fully understand the long-term effects and potential risks associated with repeated use of hypertonic fluids for drug delivery. The lack of extensive clinical data on various hypertonic formulations across different therapeutic areas remains a significant challenge in advancing this technology.
Existing Hypertonic Fluid-based Drug Delivery Solutions
01 Hypertonic fluid formulations for drug delivery
Hypertonic fluid formulations are used as carriers for drug delivery, enhancing the absorption and distribution of active pharmaceutical ingredients. These formulations can increase osmotic pressure, improving drug penetration through biological membranes and potentially enhancing therapeutic efficacy.- Hypertonic fluid formulations for drug delivery: Hypertonic fluids are used as carriers for drug delivery, enhancing the absorption and distribution of active ingredients. These formulations can improve the efficacy of various medications by creating an osmotic gradient that facilitates drug penetration into tissues.
- Controlled release systems using hypertonic solutions: Controlled release drug delivery systems utilize hypertonic solutions to modulate the release rate of active compounds. This approach allows for sustained drug delivery over extended periods, potentially reducing dosing frequency and improving patient compliance.
- Hypertonic fluids for targeted drug delivery: Hypertonic fluids are employed in targeted drug delivery systems to enhance the localization of therapeutic agents in specific tissues or organs. This method can improve treatment efficacy while minimizing systemic side effects.
- Combination of hypertonic fluids with nanocarriers: Hypertonic fluids are combined with nanocarriers to create advanced drug delivery systems. This synergistic approach can enhance drug solubility, stability, and cellular uptake, potentially improving the overall therapeutic index of various medications.
- Hypertonic solutions for transdermal drug delivery: Hypertonic solutions are utilized in transdermal drug delivery systems to enhance the permeation of active ingredients through the skin barrier. This non-invasive approach can provide an alternative to oral or injectable medications for certain therapeutic applications.
02 Controlled release systems using hypertonic fluids
Hypertonic fluids are incorporated into controlled release drug delivery systems to modulate the release rate of medications. The osmotic gradient created by these fluids can drive the release of drugs over extended periods, allowing for sustained therapeutic effects and reduced dosing frequency.Expand Specific Solutions03 Hypertonic solutions for ocular drug delivery
Specialized hypertonic solutions are developed for ocular drug delivery, enhancing the penetration of medications through the cornea and other ocular tissues. These formulations can improve the bioavailability of ophthalmic drugs and potentially reduce systemic side effects.Expand Specific Solutions04 Nasal and pulmonary drug delivery using hypertonic fluids
Hypertonic fluid formulations are utilized in nasal and pulmonary drug delivery systems to enhance absorption through mucosal membranes. These formulations can improve the local and systemic bioavailability of drugs administered via these routes, potentially offering advantages in treating respiratory conditions.Expand Specific Solutions05 Hypertonic fluid-based transdermal drug delivery
Transdermal drug delivery systems incorporating hypertonic fluids are developed to enhance skin penetration of active ingredients. These formulations can create an osmotic gradient that facilitates drug absorption through the skin, potentially improving the efficacy of topical medications and expanding the range of drugs suitable for transdermal administration.Expand Specific Solutions
Key Players in Hypertonic Fluid Research
The research on hypertonic fluids for enhancing drug delivery systems is in a growth phase, with increasing market potential due to the rising demand for targeted and efficient drug delivery methods. The global drug delivery market is projected to reach significant size, driven by advancements in biotechnology and nanotechnology. Technologically, the field is progressing rapidly, with companies like Medtronic, Novartis, and Pfizer leading innovation. Academic institutions such as Johns Hopkins University and Duke University are contributing to fundamental research, while pharmaceutical giants like Sanofi-Aventis and Bayer HealthCare are developing practical applications. The collaboration between academia and industry is accelerating the maturation of hypertonic fluid-based drug delivery technologies, promising improved therapeutic outcomes across various medical fields.
The Johns Hopkins University
Koninklijke Philips NV
Innovative Approaches in Hypertonic Fluid Formulations
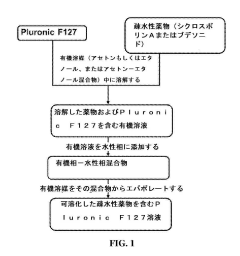
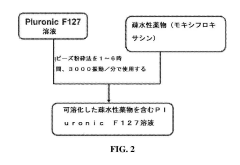
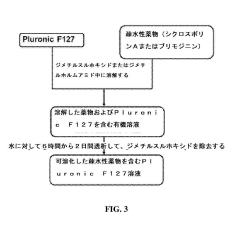
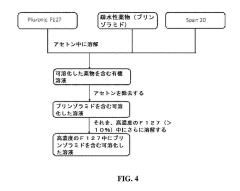
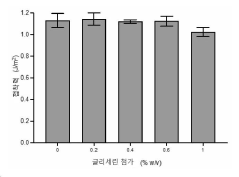
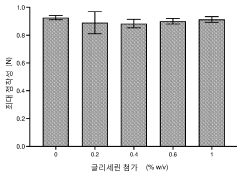
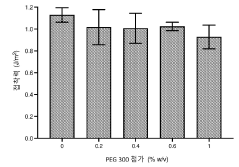
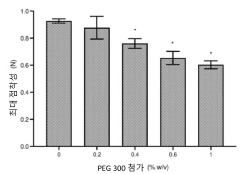
- The use of hypotonic gelling vehicles, such as poloxamers, administered below their critical gelling concentration, which absorb into mucosal tissues and form a gel at the epithelial surface, enhancing drug solubilization, distribution, and retention.
- A hypotonic gel-forming composition with low molecular weight polyethylene glycol (PEG) is added to improve viscosity and reduce adhesion, forming a uniform gel on the ocular surface for sustained drug release, using polymers like PLURONIC® F127, which maintains low viscosity for comfort and lubrication.
Regulatory Landscape for Hypertonic Drug Delivery Systems
The regulatory landscape for hypertonic drug delivery systems is complex and multifaceted, involving various governmental agencies and international bodies. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the development, approval, and marketing of these innovative drug delivery systems. The FDA's Center for Drug Evaluation and Research (CDER) is primarily responsible for evaluating the safety and efficacy of hypertonic drug formulations.
Regulatory considerations for hypertonic drug delivery systems often fall under the category of combination products, as they involve both a drug component and a delivery device. This classification necessitates a comprehensive review process that may involve multiple FDA centers, including the Center for Devices and Radiological Health (CDRH) in addition to CDER.
In the European Union, the European Medicines Agency (EMA) provides guidelines for the development and approval of novel drug delivery systems, including those utilizing hypertonic fluids. The EMA's approach emphasizes a risk-based assessment, considering both the drug substance and the delivery mechanism as integral parts of the overall product.
Regulatory bodies worldwide are increasingly recognizing the potential benefits of hypertonic drug delivery systems in improving therapeutic outcomes. However, they also acknowledge the unique challenges these systems present, such as potential tissue irritation or altered drug pharmacokinetics due to the hypertonic environment.
Safety considerations are paramount in the regulatory landscape. Manufacturers must demonstrate that the hypertonic nature of the delivery system does not adversely affect the drug's safety profile or lead to unintended physiological responses. This often requires extensive preclinical and clinical studies to evaluate local and systemic effects.
Regulatory agencies also focus on the quality and consistency of hypertonic drug delivery systems. Manufacturers must establish robust quality control measures to ensure the stability of the hypertonic formulation and the reliability of the delivery mechanism. This includes stringent testing of osmolality, pH, and other critical quality attributes throughout the product's shelf life.
As the field of hypertonic drug delivery systems evolves, regulatory frameworks are adapting to keep pace with technological advancements. Many agencies now offer accelerated review pathways for innovative therapies that address unmet medical needs, potentially expediting the approval process for novel hypertonic drug delivery systems that demonstrate significant therapeutic advantages.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in the development and application of hypertonic fluids for drug delivery systems. The use of these fluids introduces unique challenges that must be carefully addressed to ensure patient safety and treatment efficacy.
One of the primary concerns is the potential for osmotic stress on cells and tissues. Hypertonic fluids, by definition, have a higher solute concentration than physiological fluids, which can lead to cellular dehydration and shrinkage. This osmotic effect may cause damage to cell membranes, disrupt cellular functions, and potentially lead to cell death. Researchers must carefully balance the osmotic pressure of the hypertonic fluid to maximize drug delivery efficiency while minimizing cellular damage.
The choice of solutes used to create hypertonic solutions is critical for biocompatibility. Common solutes include sugars, salts, and synthetic polymers. Each of these has different interactions with biological systems and must be evaluated for their potential to cause irritation, inflammation, or allergic reactions. For instance, some sugars may be metabolized by cells, altering local biochemistry, while certain salts might disrupt ion balances crucial for cellular function.
Systemic effects of hypertonic fluids must also be considered, particularly when used for parenteral drug delivery. The introduction of hypertonic solutions into the bloodstream can cause rapid shifts in fluid balance, potentially leading to electrolyte imbalances, changes in blood pressure, and stress on the cardiovascular system. These effects can be particularly dangerous for patients with pre-existing conditions such as heart disease or kidney dysfunction.
The interaction between hypertonic fluids and the drug being delivered is another critical safety consideration. The high solute concentration may affect drug stability, solubility, or release kinetics. This could potentially alter the drug's efficacy or lead to the formation of harmful byproducts. Extensive compatibility testing is necessary to ensure that the hypertonic environment does not compromise the therapeutic properties of the drug.
Long-term effects of repeated exposure to hypertonic fluids must be evaluated, especially for chronic conditions requiring ongoing treatment. This includes assessing the potential for cumulative tissue damage, changes in local microenvironments, and the body's adaptive responses to repeated osmotic stress. Longitudinal studies are essential to understand these long-term implications and to develop appropriate treatment protocols.
Regulatory considerations for hypertonic fluid-based drug delivery systems are stringent, reflecting the complexity of these formulations. Manufacturers must provide comprehensive data on biocompatibility, toxicity profiles, and potential side effects. This often involves extensive in vitro and in vivo testing, including cell culture studies, animal models, and ultimately, carefully designed clinical trials to establish safety in humans.