How to Formulate Hypertonic Solutions for Marine Biology Studies?
Hypertonic Solutions in Marine Biology: Background and Objectives
Hypertonic solutions play a crucial role in marine biology studies, serving as essential tools for investigating osmotic processes and cellular responses in marine organisms. The development of these solutions has evolved alongside our understanding of marine ecosystems and cellular biology. Initially, researchers utilized simple salt solutions to create osmotic gradients, but as the field progressed, more sophisticated formulations emerged to better mimic natural marine environments.
The primary objective of formulating hypertonic solutions for marine biology studies is to create controlled environments that allow researchers to observe and analyze the physiological responses of marine organisms to osmotic stress. These solutions are designed to have a higher solute concentration than the intracellular fluid of the organisms under study, inducing water movement out of cells through osmosis. This process enables scientists to investigate cellular mechanisms for osmoregulation, stress tolerance, and adaptation to changing environmental conditions.
Over time, the focus of hypertonic solution formulation has shifted from basic osmotic experiments to more complex studies involving specific ion channels, membrane transport proteins, and cellular signaling pathways. This evolution reflects the growing sophistication of marine biology research and the increasing need for precise control over experimental conditions. Modern formulations often incorporate a carefully balanced mix of salts, organic compounds, and buffers to maintain pH stability and mimic the complex chemistry of seawater.
The development of hypertonic solutions has been driven by advances in analytical techniques, such as high-resolution microscopy, electrophysiology, and molecular biology. These tools have enabled researchers to observe cellular responses to osmotic stress with unprecedented detail, leading to a deeper understanding of marine organism physiology and ecology. Consequently, the formulation of hypertonic solutions has become more tailored to specific research questions and model organisms, ranging from single-celled algae to complex vertebrates.
Current trends in hypertonic solution formulation for marine biology studies include the integration of environmental factors such as temperature, pH, and dissolved gases to create more realistic experimental conditions. There is also a growing emphasis on developing solutions that can simulate future ocean conditions, particularly in the context of climate change research. These advanced formulations allow scientists to investigate how marine organisms might respond to predicted changes in ocean chemistry and salinity.
As marine biology continues to intersect with other scientific disciplines, including genetics, biochemistry, and environmental science, the formulation of hypertonic solutions is expected to become even more sophisticated. Future developments may include the incorporation of stable isotopes for tracing metabolic pathways or the use of nanoparticles for targeted delivery of osmolytes. These advancements will further enhance our ability to study the complex interactions between marine organisms and their environment, ultimately contributing to our understanding of marine ecosystem dynamics and conservation strategies.
Market Analysis for Marine Biology Research Tools
The market for marine biology research tools, particularly those related to hypertonic solution formulation, has been experiencing steady growth in recent years. This growth is primarily driven by increasing research activities in marine biology, oceanography, and related fields. The global marine biotechnology market, which encompasses various research tools and technologies, is projected to reach a substantial value by 2025, with a compound annual growth rate (CAGR) of around 8% from 2020 to 2025.
Within this broader market, the demand for specialized tools and solutions for marine biology studies, including hypertonic solutions, is showing promising trends. The increasing focus on marine ecosystem conservation, climate change impacts on marine life, and the exploration of marine resources for pharmaceutical and biotechnological applications are key factors fueling this demand.
Academic institutions, research laboratories, and marine biology centers are the primary consumers of these research tools. There is a growing need for precise and reliable methods to formulate hypertonic solutions, as these are crucial for various marine biology experiments, including osmotic stress studies, cell preservation, and physiological investigations of marine organisms.
The market is also witnessing a shift towards more sophisticated and automated solutions. Advanced equipment and software for precise solution formulation and analysis are gaining traction among researchers. This trend is partly driven by the need for reproducibility in experiments and the increasing complexity of marine biology research.
Geographically, North America and Europe dominate the market for marine biology research tools, owing to their well-established research infrastructure and significant investments in marine sciences. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing research activities in countries like China, Japan, and Australia.
The competitive landscape of this market is characterized by a mix of large scientific equipment manufacturers and specialized marine biology research tool providers. Key players are focusing on product innovation, particularly in developing user-friendly, accurate, and versatile tools for hypertonic solution formulation and related applications.
Despite the positive outlook, the market faces challenges such as the high cost of advanced research tools and the need for specialized knowledge in their operation. These factors can limit adoption, especially in smaller research institutions or developing countries. However, collaborations between academic institutions and industry players are helping to address these challenges, potentially expanding the market reach.
Current Challenges in Hypertonic Solution Formulation
The formulation of hypertonic solutions for marine biology studies faces several significant challenges that researchers and technicians must overcome to ensure accurate and reliable results. One of the primary difficulties lies in maintaining the precise osmolarity of the solution, which is crucial for studying marine organisms' physiological responses. Achieving and sustaining the desired hypertonicity requires meticulous calculation and preparation, as even slight deviations can lead to experimental errors or unintended stress on the studied specimens.
Another challenge is the selection of appropriate solutes for creating hypertonic solutions. While sodium chloride is commonly used, it may not always be suitable for all marine species or experimental conditions. Researchers must consider the specific ionic requirements of the organisms under study and the potential interactions between different solutes and biological processes. This necessitates a deep understanding of marine biochemistry and the ability to formulate custom solutions that mimic natural seawater compositions while maintaining hypertonicity.
The stability of hypertonic solutions over time presents an additional hurdle. Marine biology experiments often require extended periods, during which the osmolarity of the solution must remain constant. Factors such as evaporation, temperature fluctuations, and potential contamination can alter the solution's properties, compromising experimental integrity. Developing strategies to maintain solution stability and implementing rigorous quality control measures are essential aspects of hypertonic solution formulation.
Furthermore, the scalability of hypertonic solution preparation poses challenges, especially for large-scale studies or when working with diverse marine species. Creating consistent batches of hypertonic solutions across different experimental setups or research facilities demands standardized protocols and specialized equipment. This becomes particularly complex when dealing with rare or sensitive marine organisms that require highly specific environmental conditions.
Lastly, the potential toxicity of hypertonic solutions to marine life is a critical concern. While the goal is to study organisms under hypertonic stress, excessive or prolonged exposure can lead to cellular damage or death. Balancing the need for hypertonicity with the preservation of organism viability requires careful consideration of exposure times, gradual acclimation processes, and the development of less harmful hypertonic formulations. This challenge underscores the importance of interdisciplinary collaboration between marine biologists, chemists, and toxicologists to optimize solution formulations for specific research objectives.
Existing Hypertonic Solution Formulation Methods
01 Hypertonic solutions for medical applications
Hypertonic solutions are used in various medical applications due to their higher solute concentration compared to body fluids. These solutions can be used for treating conditions such as edema, increasing blood volume, and as a component in dialysis fluids. The tonicity of these solutions is carefully controlled to achieve the desired therapeutic effect while minimizing potential side effects.- Hypertonic solutions for medical applications: Hypertonic solutions are used in various medical applications due to their higher solute concentration compared to body fluids. These solutions can be used for treating conditions such as edema, increasing blood volume, and as a component in dialysis fluids. The tonicity of these solutions is carefully controlled to achieve the desired therapeutic effect while minimizing potential side effects.
- Osmotic regulation in cell culture media: Hypertonic solutions play a crucial role in cell culture media, where tonicity is carefully adjusted to maintain optimal cellular function. By controlling the osmotic environment, researchers can influence cell growth, metabolism, and protein expression. The use of specific solutes and their concentrations in culture media is essential for achieving desired experimental outcomes and maintaining cell viability.
- Hypertonic solutions in ophthalmic formulations: Hypertonic solutions are utilized in ophthalmic formulations to treat various eye conditions. These solutions can help reduce corneal edema, manage intraocular pressure, and provide relief for dry eye syndrome. The tonicity of these formulations is carefully balanced to ensure effectiveness while minimizing ocular irritation and maintaining patient comfort.
- Tonicity adjustment in pharmaceutical preparations: The tonicity of pharmaceutical preparations is often adjusted to match physiological conditions or to achieve specific therapeutic effects. Hypertonic solutions may be used in certain formulations to enhance drug absorption, improve stability, or control the release of active ingredients. Careful consideration of tonicity is essential in developing safe and effective drug delivery systems.
- Hypertonic solutions in diagnostic applications: Hypertonic solutions are employed in various diagnostic applications, including contrast agents for imaging studies and reagents for in vitro diagnostic tests. The tonicity of these solutions is optimized to enhance visibility, improve test sensitivity, or maintain the integrity of biological samples. The careful control of solution tonicity is crucial for obtaining accurate and reliable diagnostic results.
02 Tonicity adjustment in pharmaceutical formulations
Adjusting the tonicity of pharmaceutical formulations is crucial for ensuring their compatibility with biological systems. This process involves adding or removing solutes to achieve the desired osmotic pressure. Tonicity agents such as sodium chloride, dextrose, or mannitol are commonly used to modify the tonicity of solutions, making them suitable for various routes of administration.Expand Specific Solutions03 Hypertonic solutions in cell culture and biotechnology
Hypertonic solutions play a significant role in cell culture techniques and biotechnology applications. These solutions can be used to induce osmotic stress in cells, study cellular responses to environmental changes, and optimize conditions for protein production in bioreactors. The careful manipulation of solution tonicity is essential for maintaining cell viability and enhancing product yield in biotechnological processes.Expand Specific Solutions04 Measurement and control of solution tonicity
Accurate measurement and control of solution tonicity are critical in various fields, including medicine, pharmaceuticals, and biotechnology. Methods for determining tonicity include osmometry, freezing point depression, and vapor pressure measurements. Advanced techniques and devices have been developed to precisely measure and adjust the tonicity of solutions, ensuring their suitability for specific applications.Expand Specific Solutions05 Applications of hypertonic solutions in food and agriculture
Hypertonic solutions have applications in food preservation and agricultural practices. In food processing, these solutions can be used for osmotic dehydration of fruits and vegetables, extending their shelf life. In agriculture, hypertonic solutions may be employed in seed priming techniques to improve germination rates and seedling vigor. The controlled use of hypertonic solutions in these fields can lead to improved product quality and crop yields.Expand Specific Solutions
Key Players in Marine Biology Research Equipment
The formulation of hypertonic solutions for marine biology studies is a rapidly evolving field, currently in its growth phase. The market size is expanding due to increasing demand in aquaculture, oceanography, and marine conservation. Technologically, it's progressing from basic to more advanced stages, with universities like University of Liege and Université Catholique de Louvain leading academic research. Companies such as Genentech, Inc. and Novartis AG are driving commercial applications, while specialized firms like Laboratoire de la Mer SAS focus on marine-specific solutions. The involvement of diverse institutions, from Guangdong Ocean University to Tianjin University of Science & Technology, indicates a global effort to advance this technology, with varying levels of maturity across different applications and geographical regions.
Genentech, Inc.
Novartis AG
Innovative Approaches in Osmolyte Selection
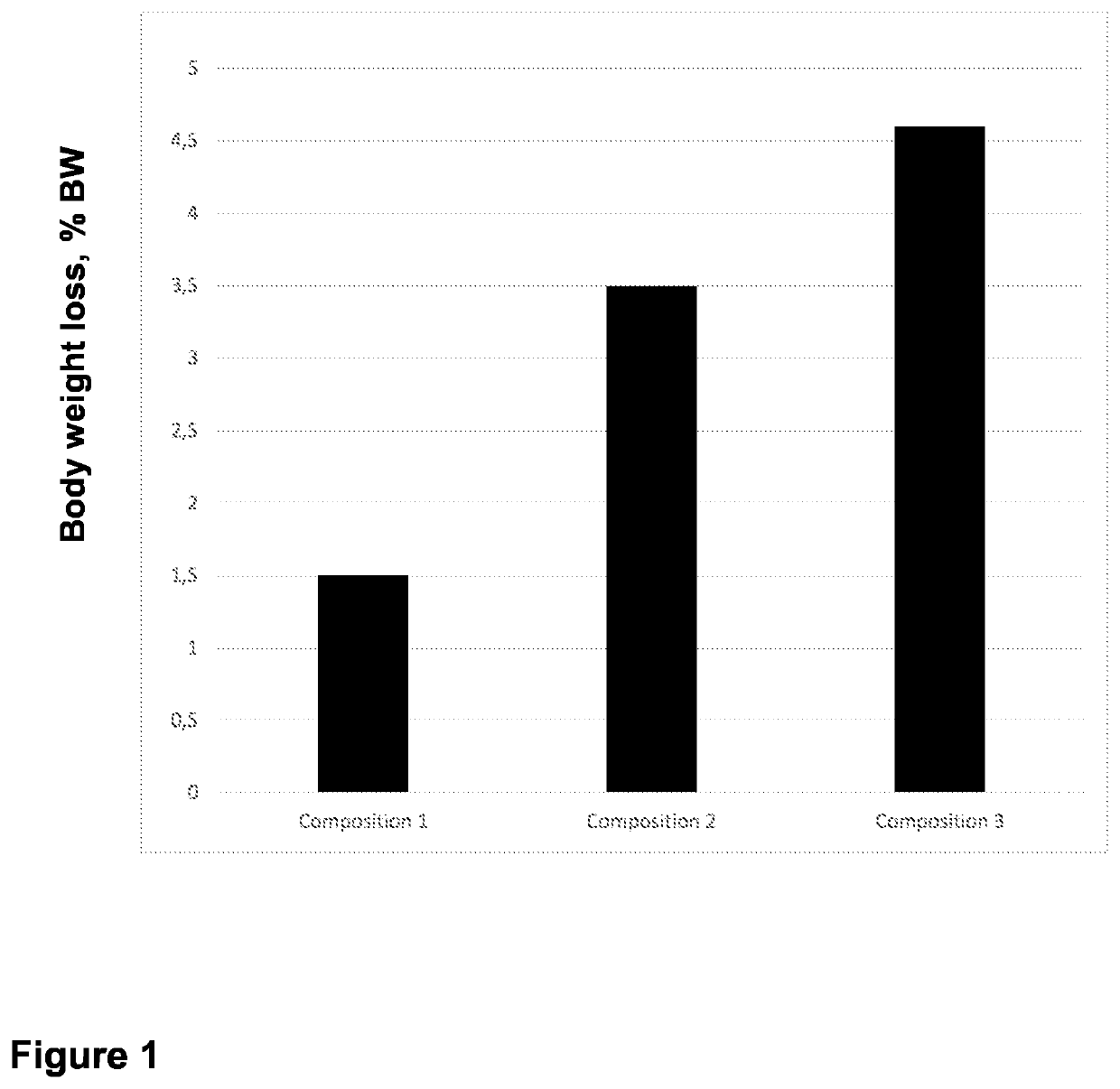
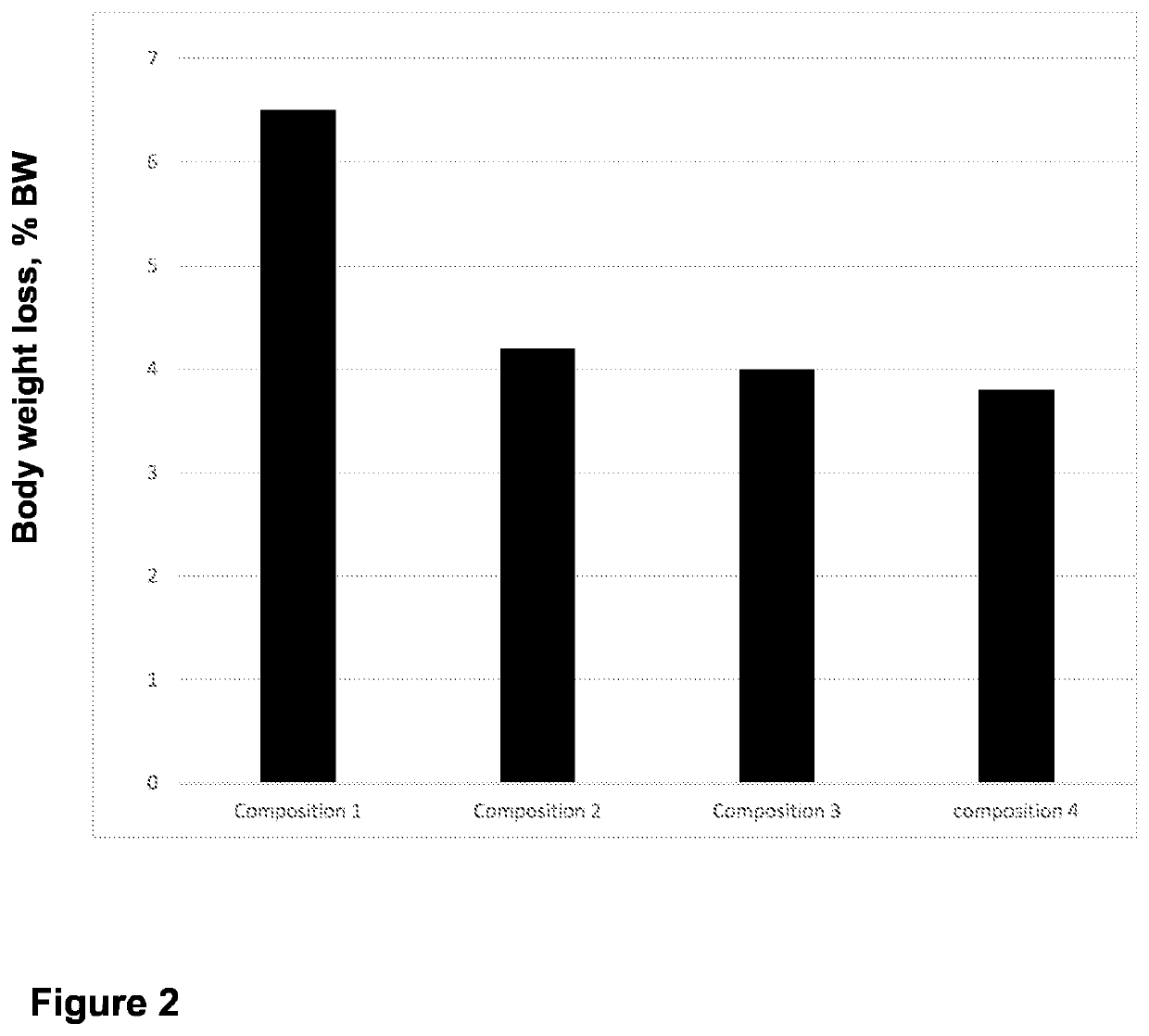
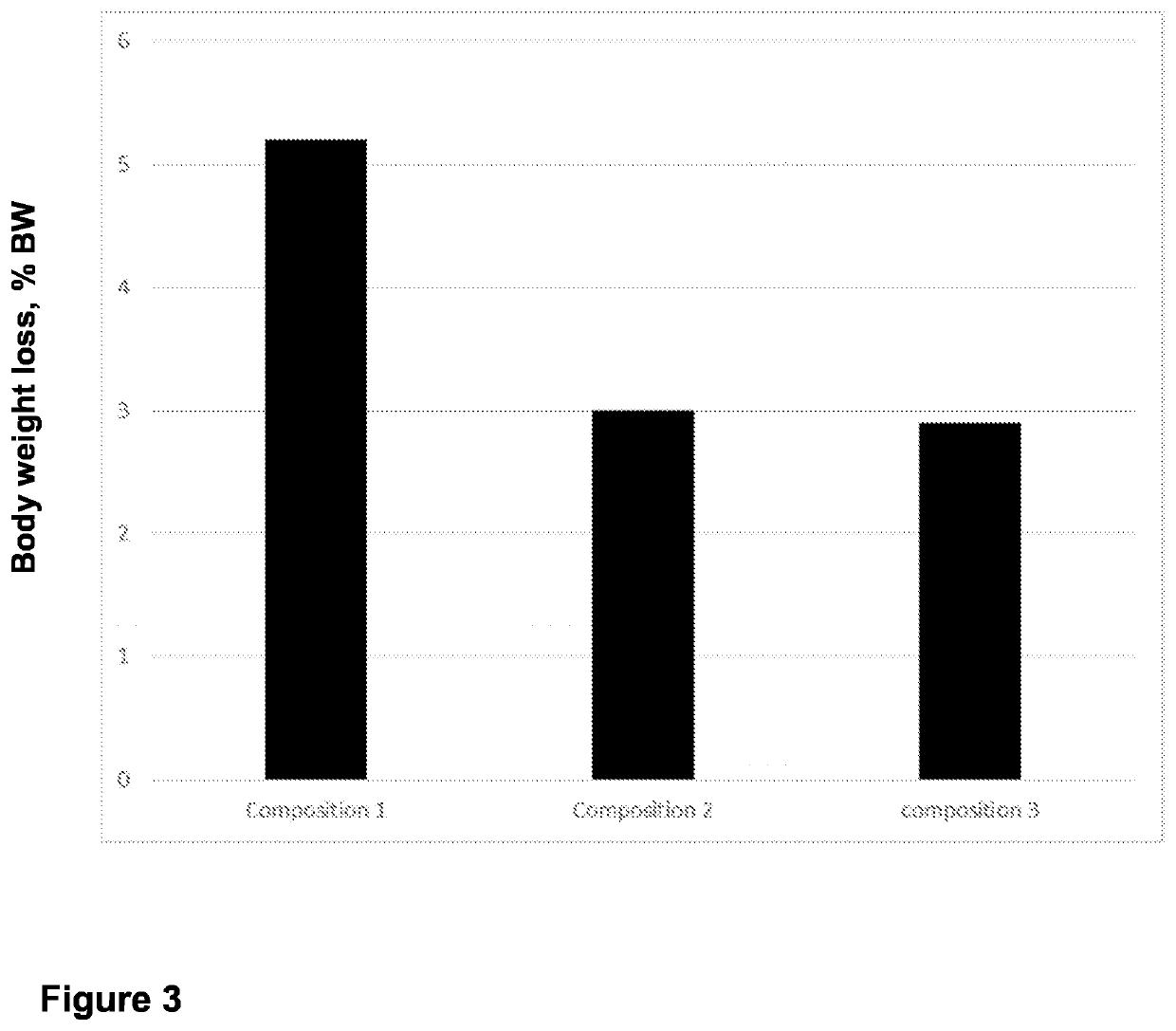
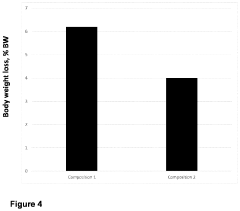
- Use of specific polymers at or above their limiting concentrations to generate osmotic pressure in hypertonic solutions for introducing macromolecules into mammalian cells.
- Utilization of polymer entanglement at limiting concentrations to enhance the effectiveness of hypertonic solutions for cellular macromolecule introduction.
- Development of a specific method for introducing macromolecules into mammalian cells using the formulated hypertonic solution.
- Aqueous compositions with a potassium to sodium ratio ranging from 65:35 to 95:05, which are hypotonic or isotonic, containing additional electrolytes like magnesium, gluconeogenic precursors like glycerol, and alkalinizing agents, administered to livestock animals during feed deprivation to maintain osmotic balance and provide energy.
Environmental Impact of Hypertonic Solutions
The use of hypertonic solutions in marine biology studies has significant environmental implications that must be carefully considered. These solutions, when introduced into aquatic ecosystems, can disrupt the delicate balance of osmotic pressure in marine organisms and their surrounding environment. The primary concern is the potential for osmotic stress on non-target species, which can lead to dehydration, cellular damage, and in severe cases, mortality.
The release of hypertonic solutions into marine environments can create localized areas of high salinity or solute concentration. This can result in the formation of density gradients, altering water circulation patterns and potentially affecting nutrient distribution. Such changes may have cascading effects on the ecosystem, influencing plankton populations, which form the base of many marine food webs.
Furthermore, the introduction of hypertonic solutions may impact water quality parameters such as pH, dissolved oxygen levels, and turbidity. These alterations can stress aquatic organisms and potentially lead to changes in species composition and biodiversity within affected areas. Sensitive species may be particularly vulnerable to these environmental shifts, potentially resulting in population declines or local extinctions.
The persistence of hypertonic solutions in the environment is another critical factor to consider. Depending on the composition of the solution and local hydrodynamics, these substances may remain in the ecosystem for extended periods, prolonging their environmental impact. This persistence can lead to chronic exposure for marine organisms, potentially causing long-term physiological adaptations or genetic changes in populations.
It is also important to consider the potential for bioaccumulation of solutes from hypertonic solutions in marine food chains. Some compounds used in these solutions may be taken up by organisms at the lower trophic levels and subsequently concentrated in predators higher up the food chain. This process could have far-reaching consequences for ecosystem health and potentially impact human food sources derived from marine environments.
To mitigate these environmental risks, researchers must implement strict protocols for the use and disposal of hypertonic solutions in marine biology studies. This includes careful selection of study sites, minimizing the volume of solutions used, and employing containment strategies to prevent unintended release into the environment. Additionally, monitoring programs should be established to assess the long-term effects of hypertonic solution use on marine ecosystems, enabling early detection of any adverse impacts and informing future best practices in marine research methodologies.
Standardization of Hypertonic Solution Protocols
The standardization of hypertonic solution protocols is crucial for ensuring consistency and reproducibility in marine biology studies. This process involves establishing uniform methods for preparing, storing, and applying hypertonic solutions across different research settings. A key aspect of standardization is the precise definition of solution concentrations, typically expressed in terms of osmolality or molarity.
Standardized protocols typically begin with the selection of appropriate solutes, such as sodium chloride, sucrose, or mannitol, depending on the specific requirements of the marine organisms under study. The exact quantities of these solutes are carefully calculated to achieve the desired osmotic pressure, taking into account the natural osmolality of seawater, which is approximately 1000 mOsm/kg.
Preparation methods are meticulously documented, including step-by-step instructions for weighing solutes, dissolving them in purified water or artificial seawater, and adjusting pH if necessary. The use of calibrated equipment, such as analytical balances and osmometers, is essential for maintaining accuracy in solution preparation.
Storage conditions for hypertonic solutions are also standardized to prevent degradation or contamination. This may include specifications for container materials, temperature requirements, and shelf-life recommendations. Additionally, protocols often include guidelines for labeling solutions with critical information such as composition, concentration, preparation date, and expiration date.
Quality control measures form an integral part of standardized protocols. These may involve regular testing of solution osmolality, pH, and sterility to ensure consistency over time. Procedures for adjusting solutions that have deviated from the standard are also included to maintain uniformity across experiments.
Application methods are standardized to ensure that hypertonic solutions are used consistently across different studies. This includes specifying the volume of solution to be applied, the duration of exposure, and any necessary pre-treatment or post-treatment steps for the marine organisms involved.
Standardization efforts also extend to data recording and reporting. Protocols typically include templates for documenting solution preparation, application procedures, and observed effects on marine organisms. This standardized documentation facilitates data sharing and meta-analysis across different research groups.
By implementing these standardized protocols, researchers can minimize variability in hypertonic solution studies, enhance the comparability of results across different laboratories, and improve the overall reliability of marine biology research involving osmotic stress experiments.