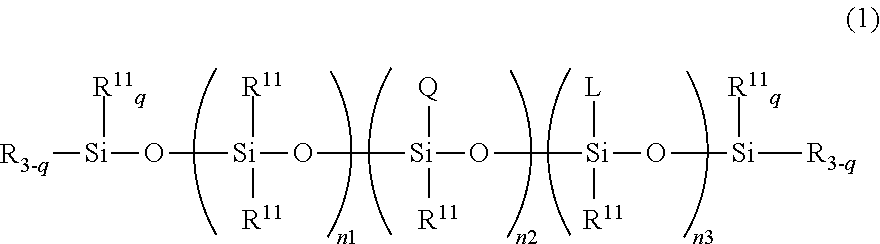Thickener or gellant for oil materials, gel composition comprising same, and method of producing cosmetic material or topical agent
a technology of oil materials and thickeners, which is applied in the direction of antibacterial agents, hair cosmetics, peptide/protein ingredients, etc., can solve the problems of poor effect exercised by organic thickeners or gellants, substantial oily feel, and heavy spreadability and tactile feel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
for Use in the Examples
Synthesis of Hydrophilic Organopolysiloxane No. 1
[0260]99.4 g of a methylhydrogenpolysiloxane with the average compositional formula MD400DH10M, 43.1 g of a vinyl-monoterminated dimethylpolysiloxane with the structural formula CH2═CHSiMe2(OSiMe2)25OSiMe3, 7.5 g of polyglycerol monoallyl ether, 150 g of isopropyl alcohol (IPA), and 0.20 g of a 2.3 weight % methanolic solution of sodium acetate were introduced into a reactor and were heated to 75° C. while stirring under a nitrogen current. 0.10 g of a 5 weight % IPA solution of chloroplatinic acid was added and a reaction was run for 7 hours at 80° C. 2 g of the reaction solution was then recovered and the completion of the reaction was confirmed through gas production method with alkali decomposition.
[0261]This reaction solution was diluted by the addition thereto of 150.0 g of dimethylpolysiloxane (2 cSt, 25° C.) with mixing. This was then heated under reduced pressure to distill out the low-boiling component...
production example 2
for Use in the Examples
Synthesis of Hydrophilic Organopolysiloxane No. 2
[0264]85.3 g of a methylhydrogenpolysiloxane with the average compositional formula MD400DH10M, 22.2 g of a vinyl-monoterminated dimethylpolysiloxane with the structural formula CH2═CHSiMe2 (OSiMe2)25OSiMe3, 6.5 g of polyglycerol monoallyl ether, 115 g of IPA, and 0.16 g of a 2.3 weight % methanolic solution of sodium acetate were introduced into a reactor and were heated to 75° C. while stirring under a nitrogen current. 0.06 g of a 5 weight % IPA solution of chloroplatinic acid was added and a reaction was run for 2 hours at 80° C. 2 g of the reaction solution was then recovered and it was confirmed by gas production method with alkali decomposition that the conversion had reached 85%. 1.0 g 1-decene and 0.06 g of a 5 weight % IPA solution of chloroplatinic acid were added and the reaction was continued for 2 hours at 80° C. When the reaction solution was then sampled again and rechecked, the reaction was foun...
example 1
Viscosification Effects of Hydrophilic Organopolysiloxane No. 1
[0310]
TABLE 210 wt %20 wt %30 wt %concentrationconcentrationconcentrationoil systemcompatibilitystatecompatibilitystatecompatibilitystate 2 cSt+viscous+gum+gum20 cSt+starch+gum+rubberysyrupSH 556+viscous+gum+gumSS-3408+viscous+gum+gum2 cSt / CEH+viscous+starch+gum(50 / 50)syrup2 cSt / IOTG+viscous+starch+gum(50 / 50)syrup2 cSt / IP+viscous+gum+gum(50 / 50)2 cSt / K-230x—+gum+rubbery(50 / 50)
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass % | aaaaa | aaaaa |
| mass % | aaaaa | aaaaa |
| mass % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com