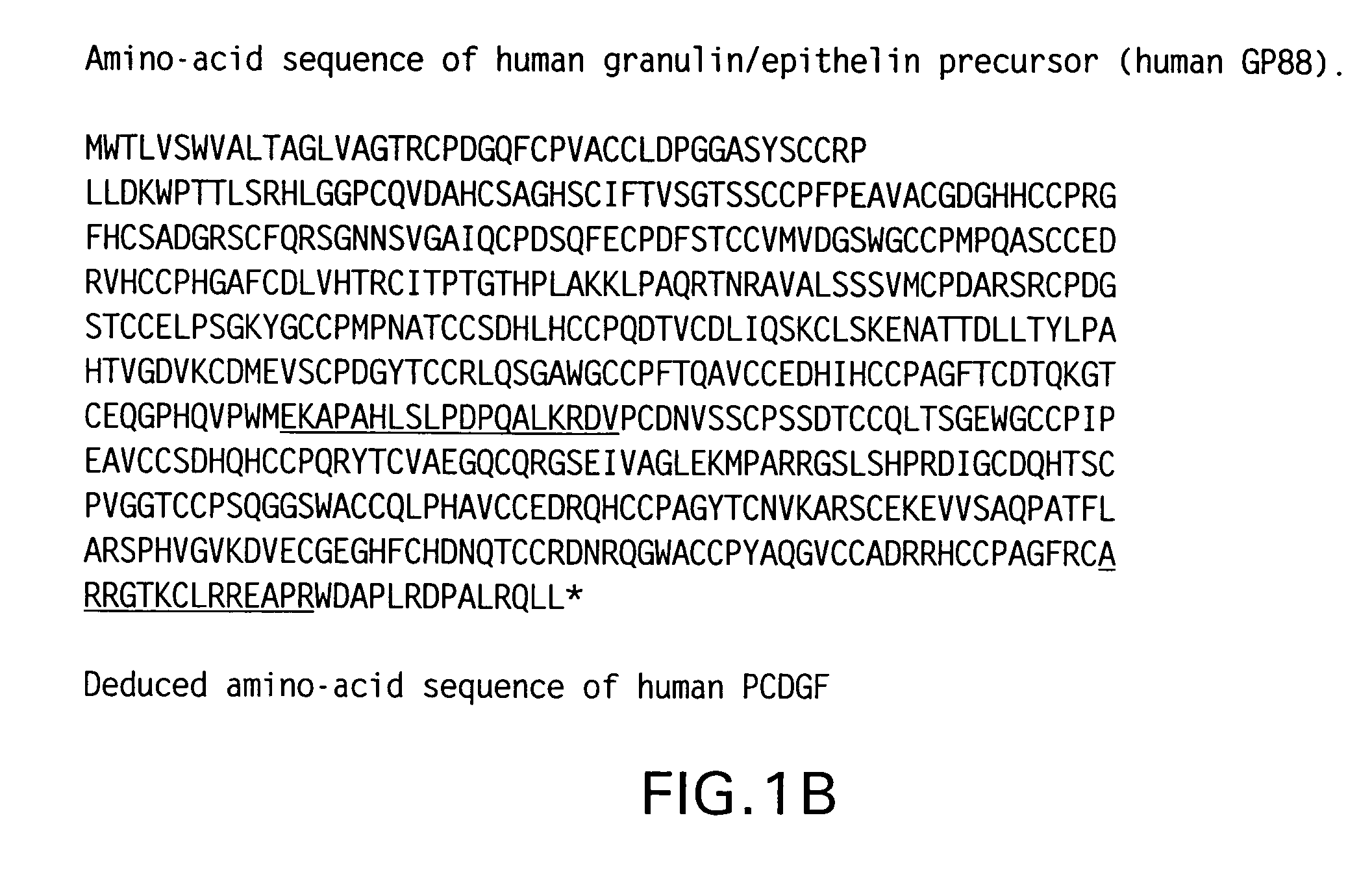
Compositions and methods for restoring sensitivity to treatment with HER2 antagonists
a technology of antagonists and compositions, applied in the field of compositions and methods for restoring sensitivity to treatment with her2 antagonists, can solve the problems of poor outcome and decreased survival of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
PCDGF Confers Resistance to the Antineoplastic Effects of HER2 Antagonist Therapy
Methods and Materials
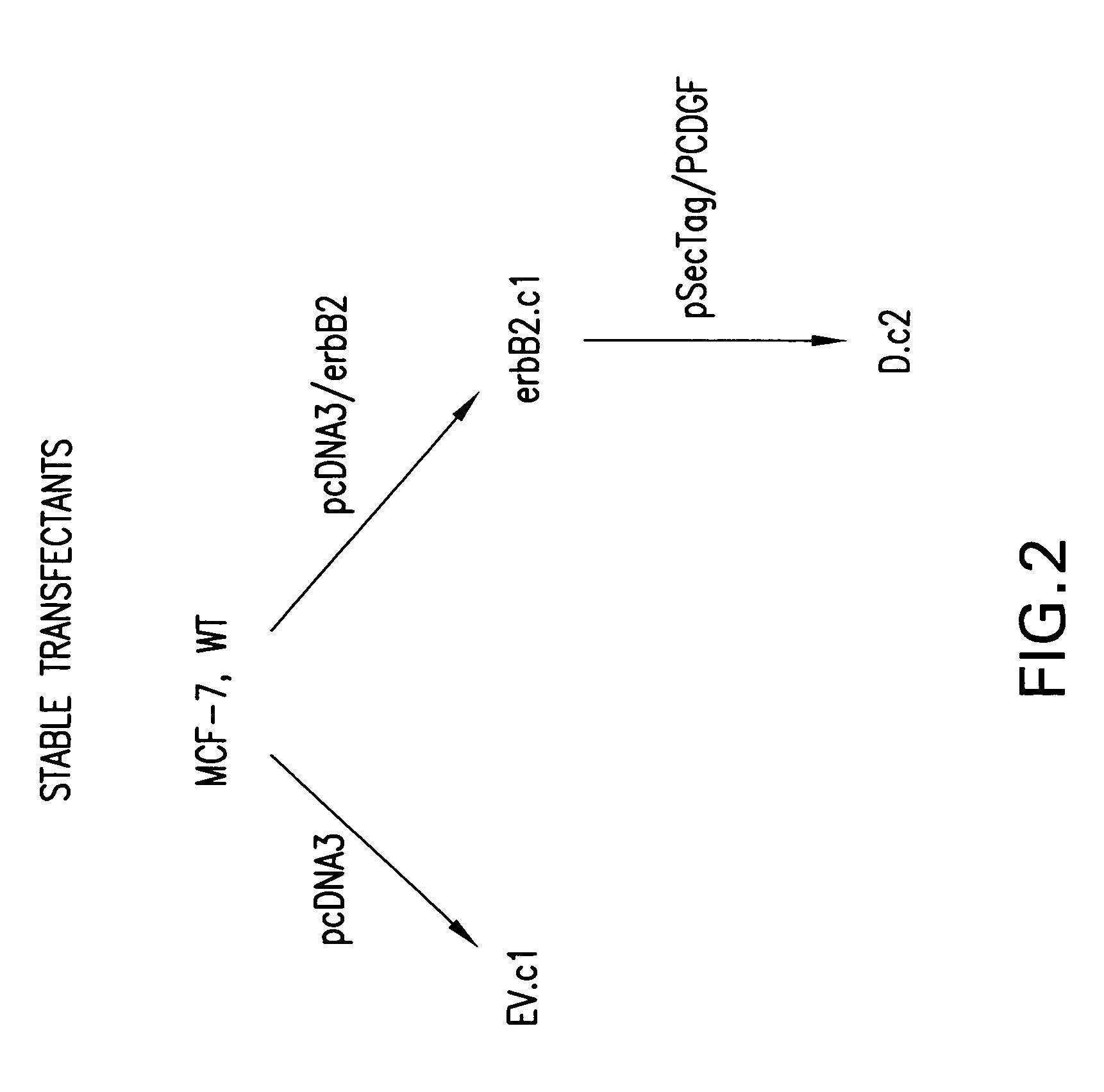
Preparation of Stable Transfectants
[0080]To investigate the role of PCDGF in HER2 antagonist resistance of c-erbB2 overexpressing cells, MCF-7 breast cancer cells were stably transfected with pcDNA3 / erbB2 expression vector (see FIG. 2) by the calcium phosphate method. A c-erbB2 overexpressing clone (erbB2.c1) was selected, and used for the subsequent stable transfection with pSecTag / PCDGF expression vector. Among the clones overexpressing both c-erbB2 and PCDGF, D.c2 clone was selected and further examined in comparison to single erbB2 overexpressing cells (see FIGS. 5, 6 and 7). Clones were established in the presence of Zeocin and / or G418.
[0081]The cells were plated in DMEF / F12 containing 5% FBS at 8×104 cells / well. After 24 hours of incubation, the cells were washed and fresh PFMEM was added along with various treatments. The cells were counted using a hem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com