Treating rotator cuff conditions
a technology for rotator cuffs and conditions, applied in the direction of peptide/protein ingredients, drug compositions, muscular disorders, etc., can solve the problems of pain and shoulder function loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
t of Damaged / Ruptured Rotator Cuff Tendons
[0039]High throughput RNA-sequencing of intact and ruptured rotator cuff tendons was performed. The sequencing analysis initially identified the BMP receptor, ACVR1C, as being highly up-regulated in intact tendons versus damaged / ruptured rotator cuff tendons (Table 1).
TABLE 1Expression of BMP receptors in rotator cuff tendon.GeneFold Change Intact vs Diseased TendonACVR13.06ACVR1B4.41ACVR1C52.41ACVR2A4.15ACVR2B2.27ACVRL10.76BMPR1A3.48BMPR1B1.57BMPR22.91
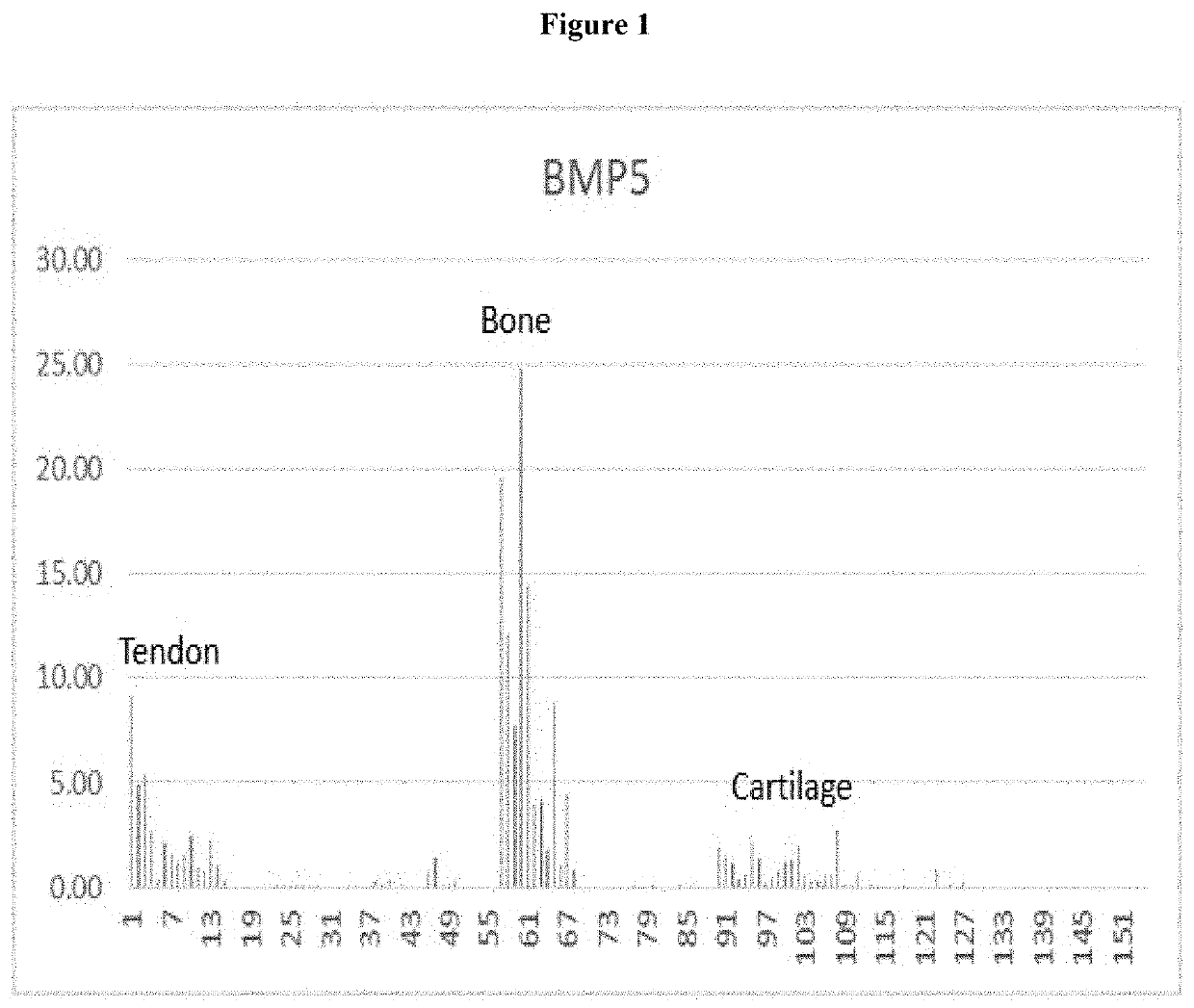
[0040]Once the ACVR1C receptor was identified, the ligands that bind to the ACVR1C receptor as well as downstream signaling molecules related to this receptor were evaluated. An evaluation of sequencing data revealed that BMP5 exhibited the greatest increase in expression amongst the possible ACVR1C receptor ligands, showing an 11.06 fold increase in intact rotator cuff tendon compared to diseased ruptured tendons (Table 2). A decrease in BMP signaling in damaged rotator cuff tendon also was c...
example 2
BMP-5 Polypeptides to Treat Damaged / Ruptured Rotator Cuff Tendons
[0044]BMP-5 polypeptides are synthesized at GMP-grade facilities using eukaryotic cells lines (e.g., CHO cells, HeLa cells, or adipose derived mesenchymal stem cells) to produce BMP-5. The BMP-5 is purified from cell extracts. 0.5 to 10 mg of BMP-5 polypeptide is formulated with 1 to 10 mL of 4 mM HCl or other soluble solution to obtain a liquid composition having a final concentration of about 0.5 to 5 mg / mL. This liquid composition is injected at a dose of 0.1 to 5 mg / mL directly into degenerative or surgically repaired tendons or into the joint space to deliver a total amount of BMP-5 in the range of 0.1 mg and 50 mg per administration.
example 3
Cells Designed to Express BMP-5 Polypeptides to Treat Damaged / Ruptured Rotator Cuff Tendons
[0045]A viral vector or other nucleic acid-based vector designed to express BMP-5 constitutively in a cell is introduced into stem cells or other somatic cell types. These modified cells are formulated with saline or other cell culture medium to obtain a liquid composition having a final concentration of about 100,000 to 20×106 cells / mL. This liquid composition is injected at a dose of about 100,000 to 20×106 cells / mL directly into a damaged tendon or a joint / subacromial space to deliver a total number of cells in the range of 100,000 and 20×106 per administration. BMP-5 expressing stem cells are injected with or without ultrasound guidance. In some cases, these techniques can be used to treat degenerative tendons at risk for rupture or to augment surgical repair of previously ruptured tendons.
Other Embodiments
[0046]It is to be understood that while the invention has been described in conjunct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com