Recipe for the synthesis of metastable structures using topologically assembled precursors
a topologically assembled and precursor technology, applied in the direction of single layer graphene, chemical property prediction, instruments, etc., can solve the problems of suppressing many predicted metastable structures are dismissed as hard-to-realize, etc., to suppress the accessibility of structures of the ground state, increase the volume of potential wells of metastable structures, and enhance the realizability of targeted metastable structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
etails of the Method Steps
[0106]1. Degrees of Freedom of the Precursor as a Rigid Body
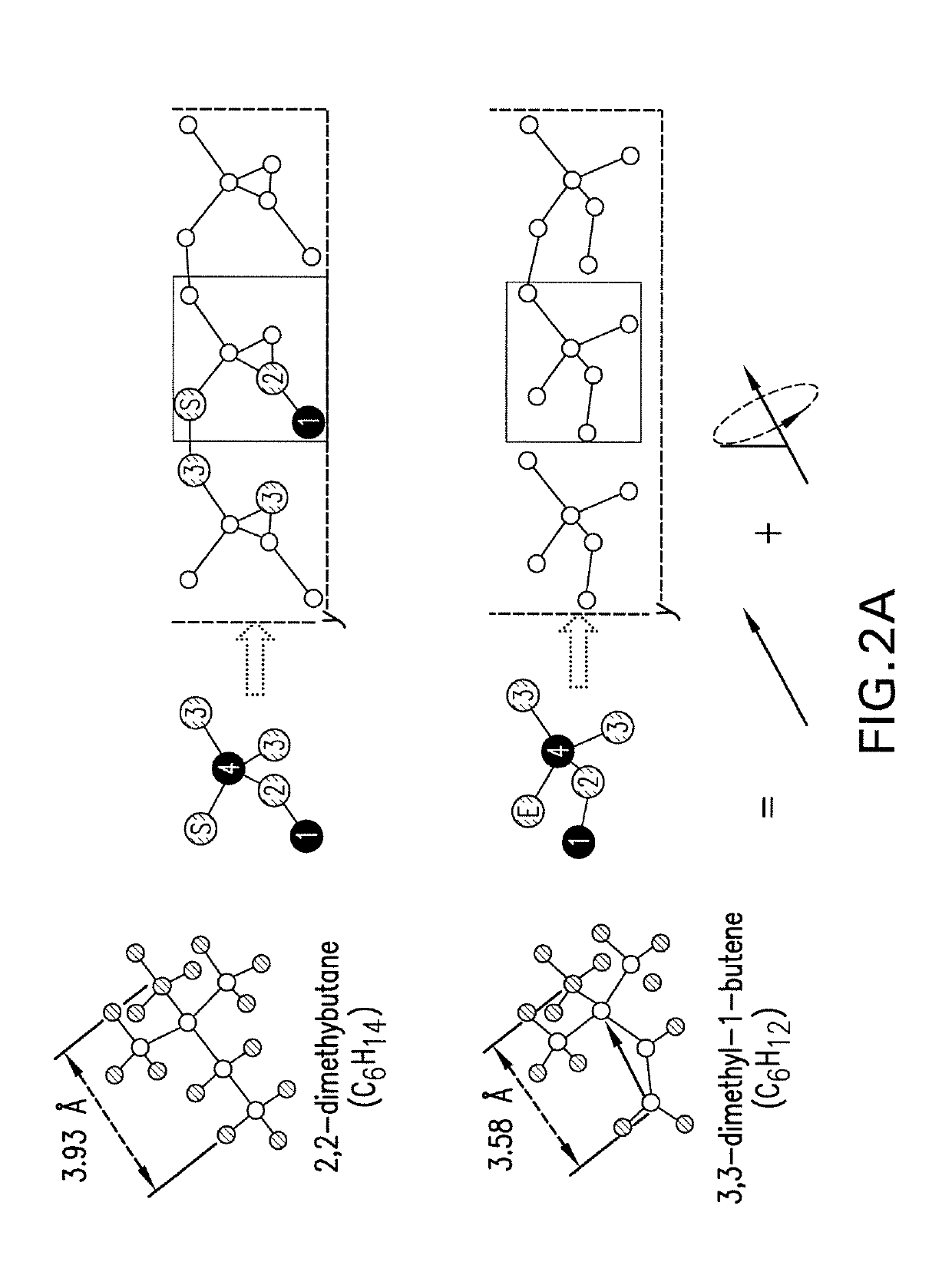
[0107]A rigid body of the 3,3-dimethyl-1-butene has three rotational degrees of freedom and the three chosen rotational angles in the paper are considered as a good description. The six carbon atoms in the molecule have 3×6=18 degrees of freedom. There are 5 fixed bonds and 7 fixed angles between the carbon atoms. Therefore, the net degrees of freedom are 18−5−7=6, three of which are rotational degrees of freedom and the other three are translational. Only the rotational degrees of freedom are important herein, since only the orientation of the precursor molecules is considered. Thus, the three rotational degrees of freedom can be chosen as the two angles that can determine the orientation of a dipole vector and the third one as the rotational angle about the dipole vector.
2. Computational Methods to Generate FIG. 3 and FIG. 4 of Example 1
[0108]The work flow of computation is given in FIG. 5. 1000 ...
example 3
of Penta-Graphene Using 3,3-dimethyl-1-butene as a Precursor
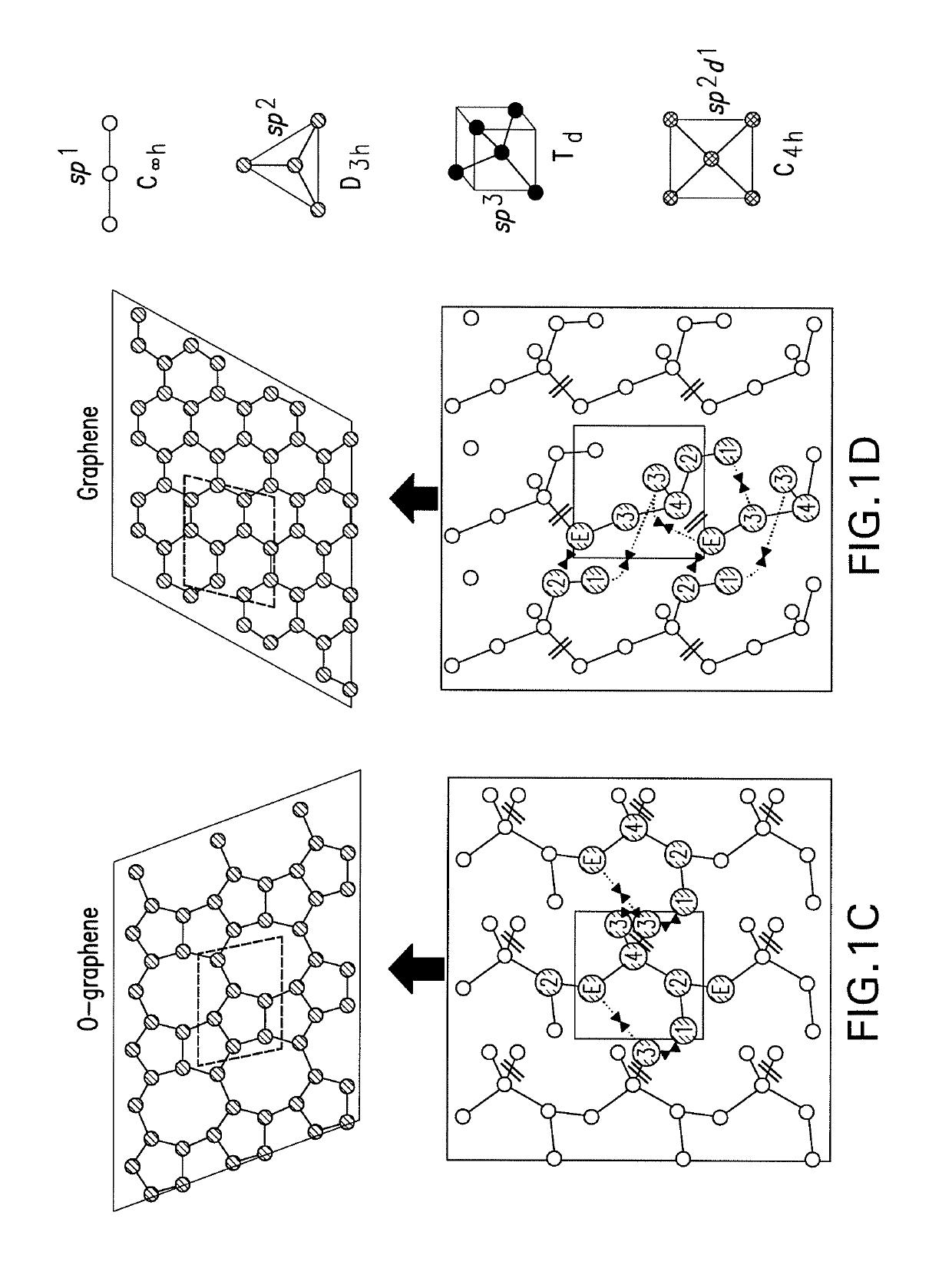
[0112]According to the steps described in Example 1, PES is constructed using TAP of 3,3-dimethyl-1-butene units, as shown in FIG. 4. On the PES, it is shown that penta-graphene exists in those striped areas with large γ values between the two high ridges, away from the depressions. The optimal condition to make penta-graphene is found to be around β=10°, where a basin of penta-graphene with a significant range [α=−10°1 state representing aninterconversion barrier of 0.30 eV / atom. On its upper-left is a shallow depression corresponding to D-graphene comprised of three-, five- and ten-membered rings whose energy is 0.02 eV / atom higher. A structure of graphene with carbynes (linear chains of carbon) appears in a small-opening dip, which is isolated by wide surrounding peaks over 1.00 eV / atom. Thus, penta-graphene can be synthesized using the precursor 3,3-dimethyl-1-butene with TAP constructed in the orientations measured by ...
example 4
Select Precursors Using Computer Program
[0114]1. Import the crystal structure of the targeted metastable compounds (e.g. the periodic structure of penta-graphene by inputting its lattice parameters and atomic coordinates).
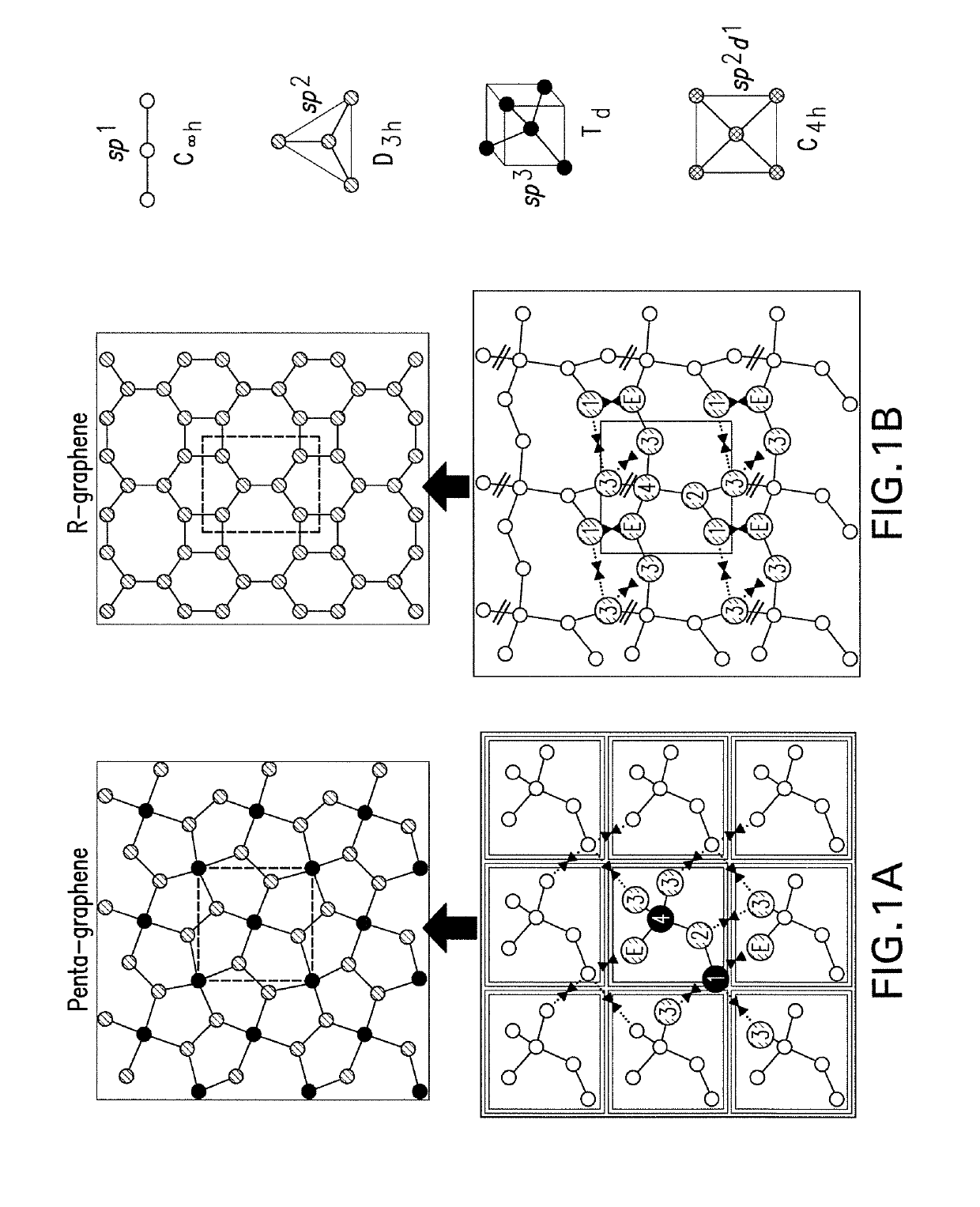
2. Read in the lattice vectors in three dimensions (length of the edge along each axis and the angle between the lettice vectors) and the number of atoms in each unit cell of the imported structure. Find the inequivalent atoms (in terms of the type and the local bonding symmetry, or the coordination number) in each unit cell (repetitive unit in the periodic structure). For example, in the case of penta-graphene, find that there are two sp3 (four coordinated) carbon atoms and four sp2 (three coordinated) carbon atoms in each unit cell, where each sp3 carbon is coordinated by four sp2 carbons.
3. Scan the database of molecular compounds for the selection of precursor using the following criteria: a) the total number of atoms in the molecule should match or be divisibl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| β | aaaaa | aaaaa |
| β | aaaaa | aaaaa |
| energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com