PROCESS FOR PREPARATION OF SECRETORY IgA AND SECRETORY IgM
a technology which is applied in the field of process for the preparation of secretory iga and secretory igm, can solve the problems of further disruption of the intestinal flora, 20-25% incidence of disease relapse, and difficulty in isolation of secretory forms of immunoglobulins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
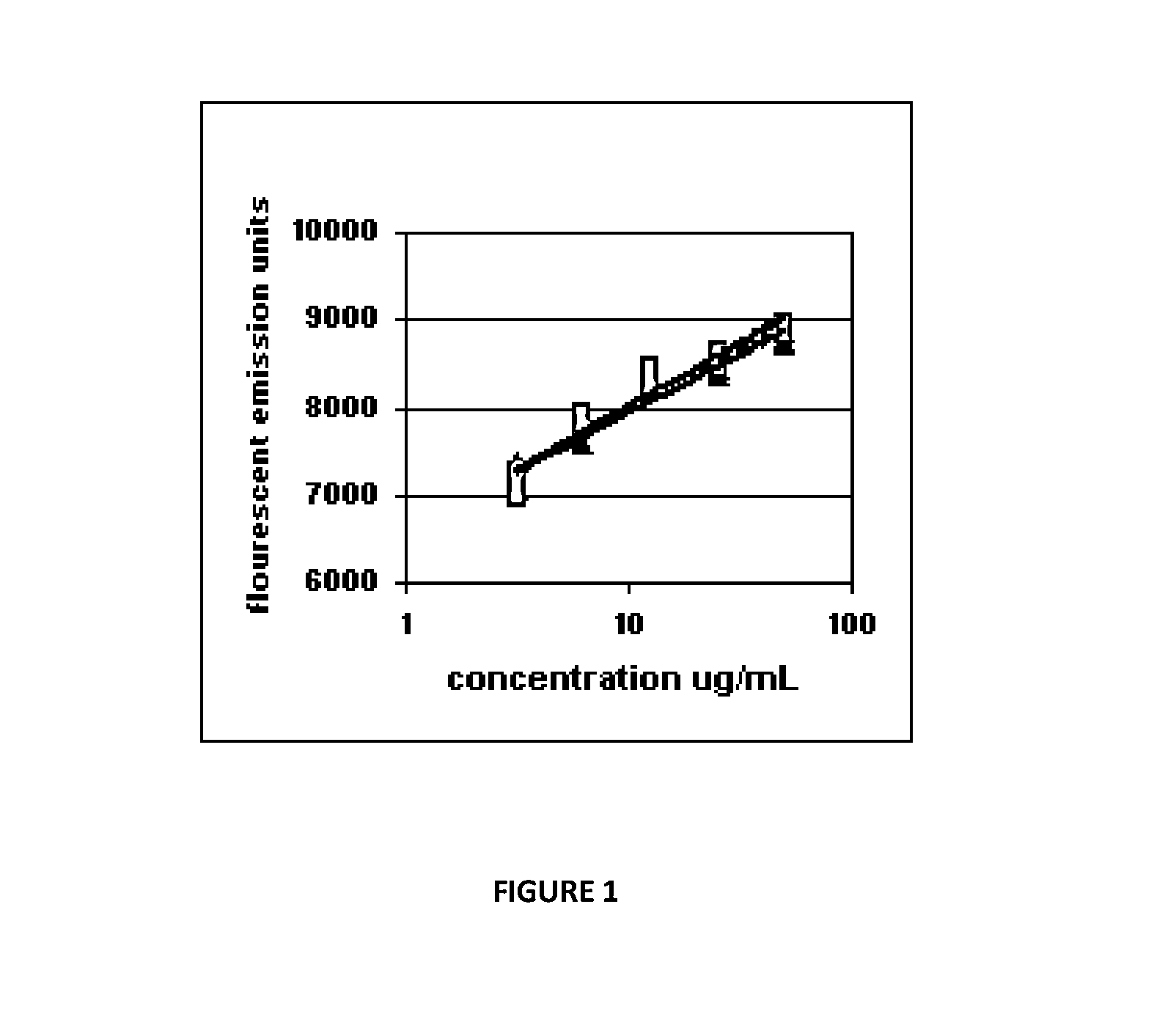
[0019]The present invention has utility for the preparation of secretory IgA or secretory IgM. In some inventive embodiments, the IgA is derived from a mixture of monomeric and dimeric plasma IgA. or in other inventive embodiments, the preparation of secretory IgM is derived from a mixture of IgM with other plasma proteins. The present invention is superior to monomeric IgA or pentameric IgM administered orally because the presence of secretory component protects the IgA or IgM from digestion in the gastrointestinal tract. Without intending to be bound to particular theory, it is believed that the increased efficacy of the present invention is achieved for secretory IgA or secretory IgM owing to the propensity of monomeric IgA and pentameric IgM to degrade in the gastrointestinal tract. The resultant dosing requirements increase treatment costs. While the present invention is further detailed principally with respect to IgA, it is appreciated that the process and medicaments that re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com