After an MI, a patient is at
increased risk for experiencing potentially life-threatening abnormal heart rhythms, or arrhythmias.
This
increased risk is caused by numerous structural and electrical abnormalities in the recently damaged heart.
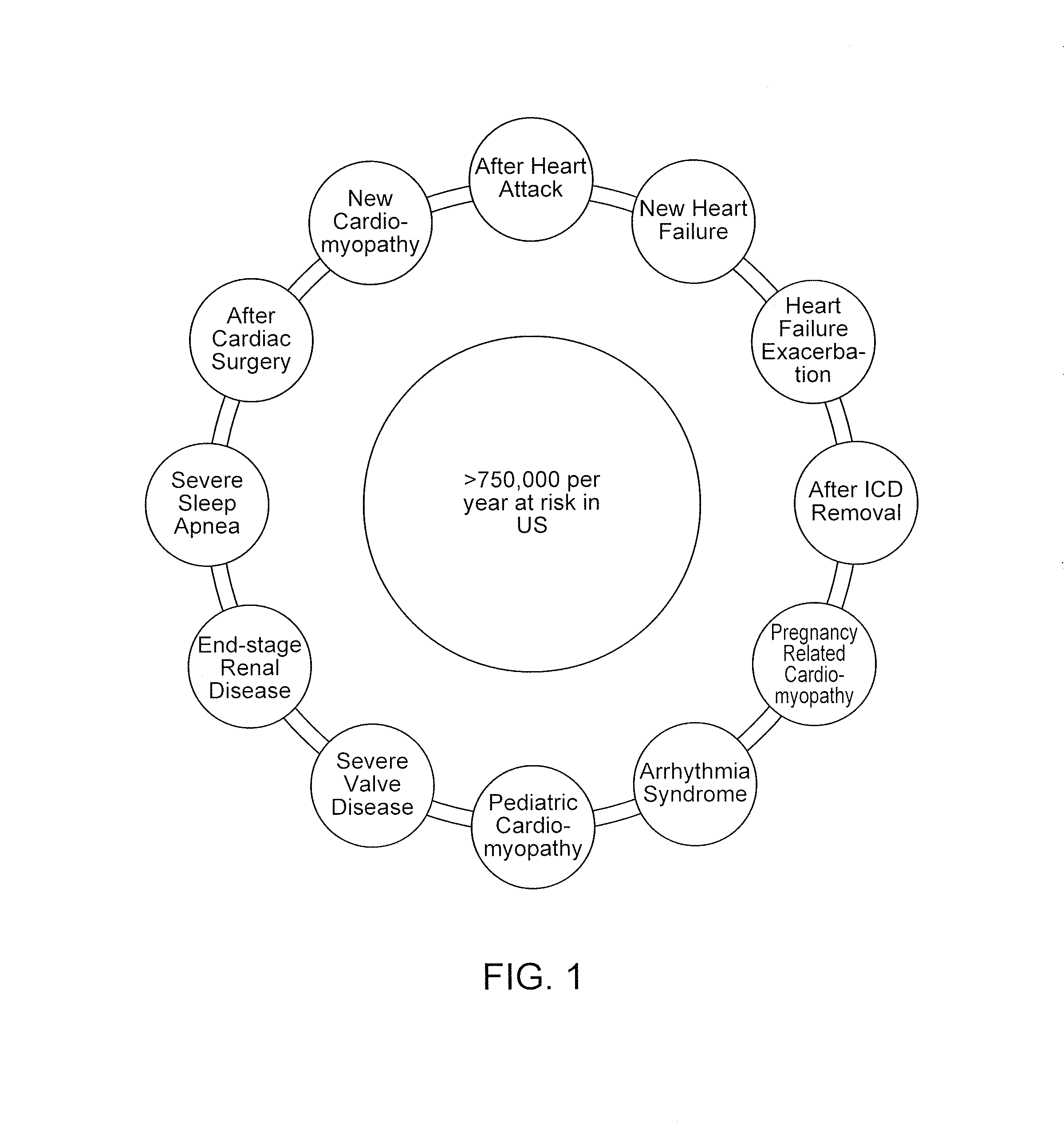
In patients who are known to be at risk for an arrhythmia and who have an ICD or S-ICD in place, if the ICD / S-ICD needs to be removed for a short period of time due to an infection or malfunction, the patient is also left vulnerable.
However, during the time of treatment when heart function is recovering or when the patient is receiving treatment, these patients are still temporarily at risk for a life-threatening arrhythmia.
Various studies of this
population of patients have shown that certain medications, especially those with anti-arrhythmic properties, do a poor job at reducing this temporarily increased arrhythmia risk.
Implantable cardioverter defibrillators (ICDs) and subcutaneous ICD (S-ICDs), which can continuously monitor the patient for an arrhythmia and effectively reset the heart
rhythm when an arrhythmia occurs, carry significant risks during implantation such that their overall benefit during this short period of increased risk is limited.
One drawback of currently available wearable defibrillators (such as the LifeVest product) is lack of
patient compliance.
Because of the size, shape and weight of these wearable devices, patients are reluctant to wear them due to discomfort, their bulkiness under clothes or limitations in the devices themselves.
In particular, such devices cannot be worn in the
shower or bath, and they often are difficult, if not impossible, to sleep in.
The device therefore is not useful in providing treatment to the patient while sleeping or in the
shower.
Patients also complain that the LifeVest is too large and uncomfortable.
Many patients also have increased
anxiety over the many alarms and notifications from the LifeVest.
The increased
anxiety further increases instances of non-compliance.
Given the bulkiness of these devices, some patients do not like using these wearable devices outside in public as it draws unnecessary attention to them, which they might find uncomfortable or embarrassing.
This may affect their well-being and may lead them to avoid performing their normal routine activities.
All of these factors increase patient noncompliance and prevent the treatment of a treatable arrhythmia.
Another drawback is that it is possible to incorrectly wear a wearable
vest like the LifeVest, such that the
vest will not properly detect a patient arrhythmia.
The design of the
vest can also result in increased false positives of arrhythmias measured by the vest.
The vest also has a complicated
electrode design.
The gel releasing mechanism can fail or may not work when the vest is worn incorrectly.
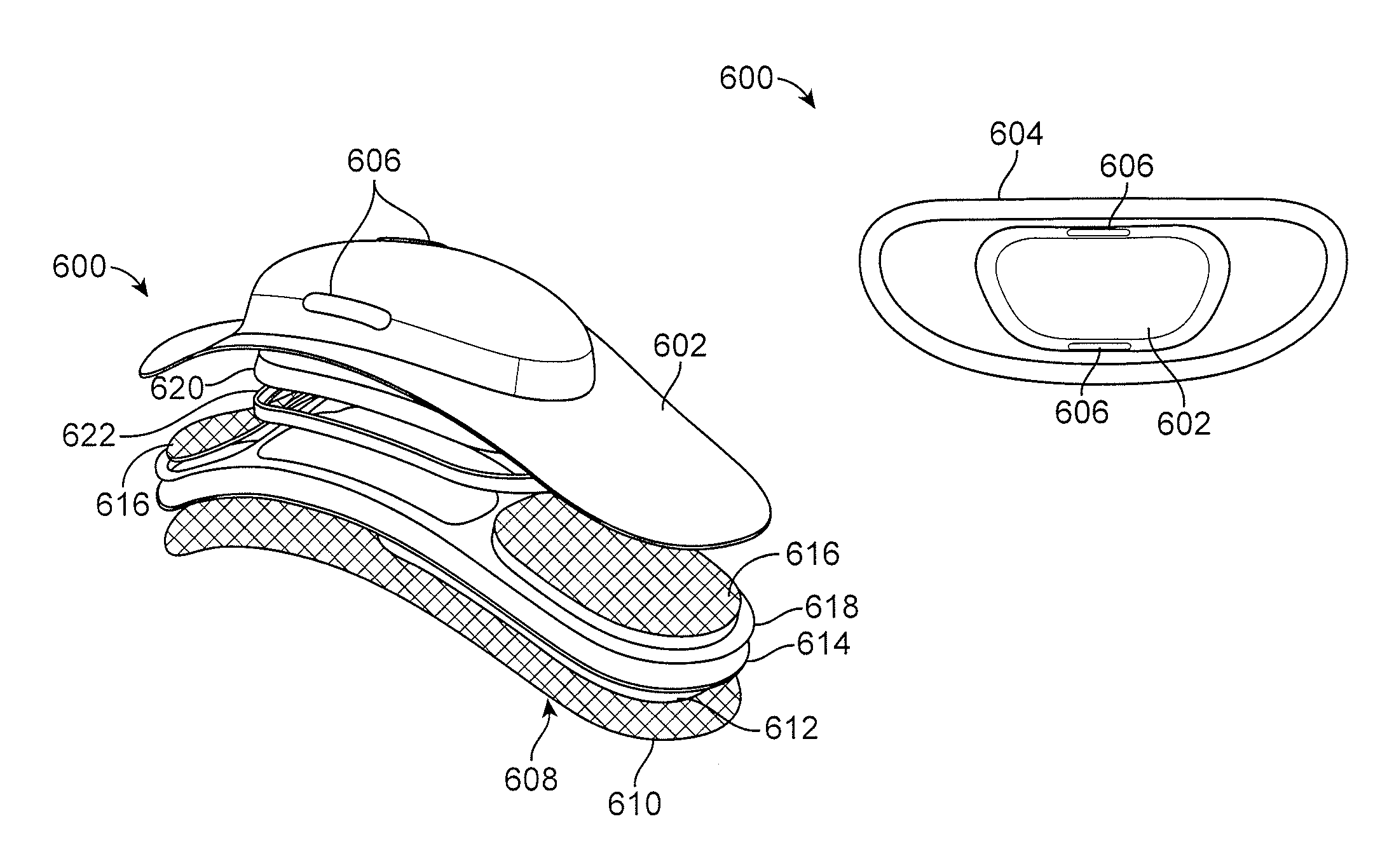
There are many challenges in developing biocompatible adhesives and electrodes for long-term wear.
It is difficult to design adhesives that can be worn for longer than 10 days.
The natural sloughing of
skin cells also presents technical challenges that need to be solved by the design of the
adhesive material and design of the electrodes.
Adhesives and electrodes also typically will cause
skin irritation and redness during long term wear.
Developing a device that also is small enough to allow a
weight distribution while adhered to the patients such that the device can be used constantly for long term wear is a challenging task.
Additionally, developing a device small enough to be concealed such that its use in public does not draw attention or can be easily hidden under normal clothing is desired.
 Login to View More
Login to View More  Login to View More
Login to View More