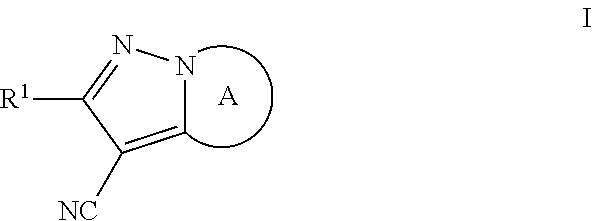
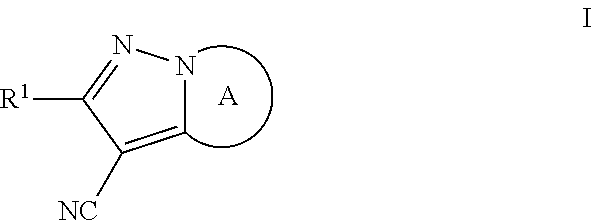
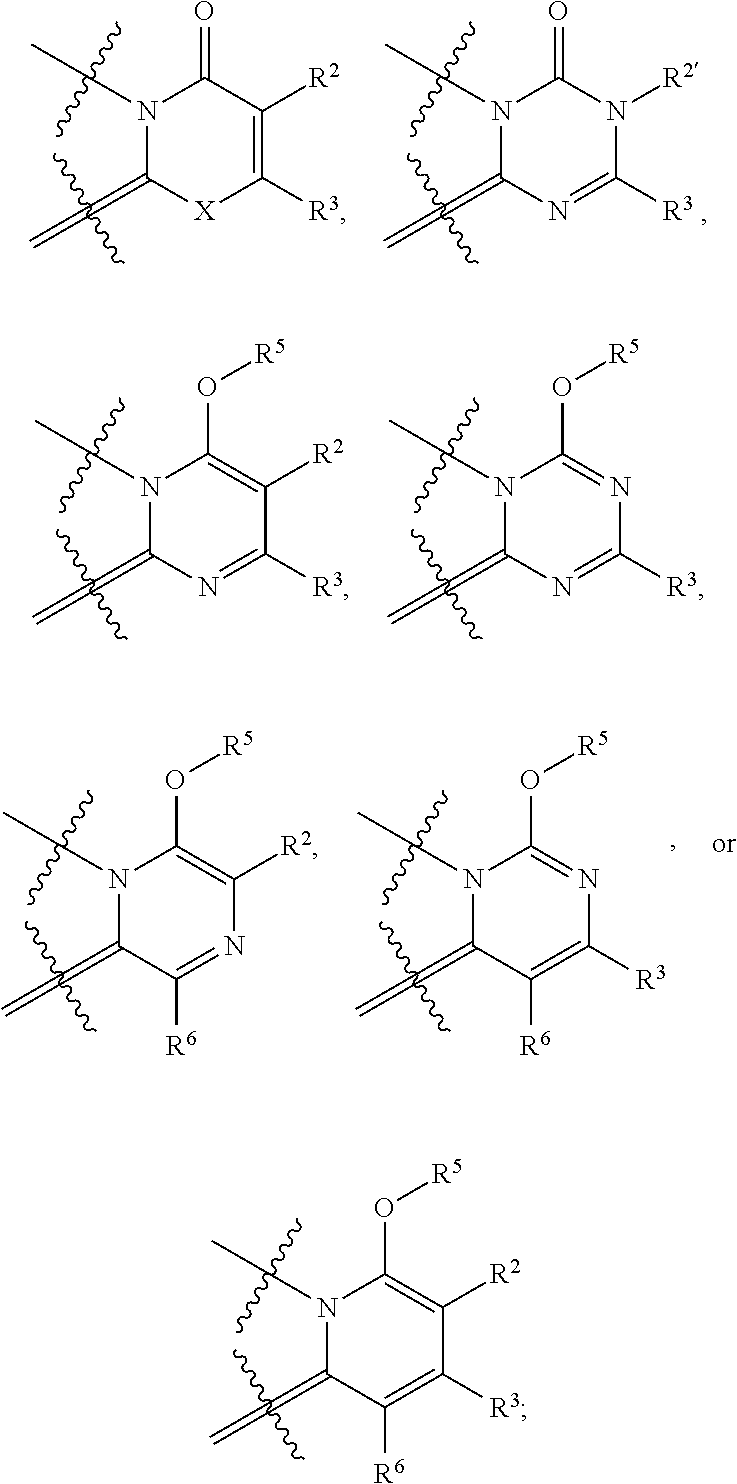
Pyrazolo compounds and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of ethyl 2-acetylpentanoate
[0159]
[0160]Ethyl 2-acetylpentanoate was synthesized according to Beddow et al, Org. Biomol. Chem. 5: 2812-2825 (2007). To a solution of t-BuOK (11.8 g, 0.11 mol) in THF (150 mL) was added ethyl 3-oxobutanoate (13 g, 0.1 mol) dropwise at 0° C., after stirred for 30 minutes, 1-bromopropane (12.3 g, 0.1 mmol) was added dropwise and the mixture was refluxed for 16 h. The reaction mixture was quenched by water, extracted with EtOAc (100 mL×2), combined organic layer was dried over anhydrous Na2SO4, evaporated, purified by column chromatography to give the expected compound ethyl 2-acetylpentaoate (8.5 g, 49%) as colorless oil. m / z (ESI) 173 [M+H]+.
example 2
[0161]By a similar method to Example 1, using the appropriate starting materials, the compounds in Table 2 were prepared.
TABLE 2Compound nameStructureDataethyl 2-acetyl-4- methylpentanoatem / z (ESI) 187 [M + H]+ethyl 2- acetylpent-4- enoatem / z (ESI) 171 [M + H]+ethyl 2-acetyl-4- methoxybutanoatem / z (ESI) 189 [M + H]+ethyl 2- acetylpent-4- ynoatem / z (ESI) 169 [M + H]+ethyl 2-ethyl- 4,4,4-trifluoro-3- oxobutanoatem / z (ESI) 213 [M + H]+ethyl 4-ethoxy-2- ethyl-3- oxobutanoatem / z (ESI) 203 [M + H]+ethyl 2-(2- methoxyacetyl) pent-4-enoatem / z (ESI) 201 [M + H]+ethyl 2- benzoylbutanoatem / z (ESI) 221 [M + H]+ethyl 2-(furan-2- carbonyl) butanoatem / z (ESI) 211 [M + H]+3-ethylpentane- 2,4-dionem / z (ESI) 129 [M + H]+
example 3
Synthesis of ethyl 2-ethyl-4-methoxy-3-oxobutanoate
[0162]
[0163]Ethyl 2-ethyl-4-methoxy-3-oxobutanoate was prepared according to WO 98 / 43968. Zinc (2 g, 30 mmol), methoxyacetonitrile (1.42 g, 20 mmol) and a catalytic amount of mercuric chloride in toluene (50 mL) were heated to reflux. Ethyl 2-bromobutanoate (5.85 g, 30 mmol) was added dropwise, then reflux continued for a hour, and cooled to a room temperature. 10% Aqueous sulfuric solution (16.5 mL) was added, and the organic layer was separated. The aqueous layer was further extracted with ethyl ether and the combined organic layers washed with water and saturated sodium bicarbonate solution, then dried over anhydrous magnesium sulfate and concentrated in vacuo. The residue was purified by column chromatography to give the product as yellow oil (1.7 g, 45%). 1H NMR (300 MHz, CDCl3) δ 4.14 (q, J=7.2 Hz, 2H), 4.07 (d, J=3.6 Hz, 2H), 3.45 (t, J=7.2 Hz, 1H), 3.37 (s, MI), 1.85 (m, 2H), 1.22 (t, J=7.2 Hz, 3H). 0.90 (t, J=7.5 Hz, 3H); m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com